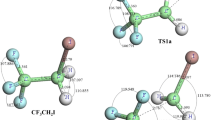
Abstract
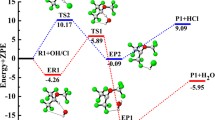
Theoretical investigations were carried out on the multi-channel reactions CF3 + SiHF3, CF3 + SiHCl3, CH3 + SiHF3, and CH3 + SiHCl3. Electronic structures were calculated at the MP2/6-311+G(d,p) level, and energetic information further refined by the MC-QCISD (single-point) method. The rate constants for major reaction channels were calculated by the canonical variational transition state theory with small-curvature tunneling correction over the temperature range of 200–1,500 K. The theoretical rate constants were in good agreement with the available experimental data and were fitted to the three parameter expression: k 1a(T) = 2.93 × 10−26 T 4.25 exp (−318.68/T), and k 2a(T) = 3.67 × 10−22 T 2.72 exp (−1,414.22/T), k 3a (T) = 7.00 × 10−24 T 3.27 exp (−384.04/T), k 4a(T) = 6.35 × 10−22 T 2.59 exp (−603.18/T) (in unit of cm3molecule−1s−1) are given. Our calculations indicate that hydrogen abstraction channel is the major channel due to the smaller barrier height among four channels considered.

Theoretical investigations on the reaction mechanisms of SiHX3 with CF3 and CH3 radicals. Rate constants were calculated in the temperature range 200―1,500 K. Our calculations indicate that hydrogen abstraction is the major channel, and is important in a wide variety of materials synthesis processes, in glow discharge deposition of amorphous silicon films, and in the semiconductor manufacturing process







Similar content being viewed by others
References
Fester GW, Eckstein J, Gerlach D, Wagler J, Brendler E, Kroke E (2010) Inorg Chem 49:2667–2673
Giraldo OH, Willis WS, Márquez M, Suib SL, Hayashi Y, Matsumoto H (1998) Chem Mater 10:366–371
Valente G, Cavallotti C, Masi M, Carrà S (2001) J Cryst Growth 230:247–257
Reznik B, Gerthsen D, Zhang WG, Hüttinger KJ (2003) J Eur Ceram Soc 23:1499–1508
Yang Y, Zhang WG (2009) Chin J Chem Eng 17:419–426
Zhang P, Wang WW, Cheng GH, Li JL (2011) Chin J Chem Eng 19:1–9
Schlegel HB (1984) J Phys Chem 88:6254–6258
Zhang WG, Hüttinger KJ (2001) Chem Vap Depos 7:173–181
Lee JY, Lee WH, Park YK, Kim HY, Kang NY, Yoon KB, Choi WC, Yang OB (2012) Sol Energy Mater Sol Cells 105:142–147
Koinuma H, Manako T, Natsuaki H, Fujioka H, Fueki K (1985) J Non-Cryst Solids 77:801–804
Matsuda A, Yagii K, Kaga T, Tanaka K (1984) Jpn J Appl Phys 23:L576–L578
Robertson R, Hils D, Gallagher A (1984) Chem Phys Lett 103:397–404
Longeway PA, Estes RD, Weakliem HA (1984) J Phys Chem 88:73–77
Kerr JA, Slater DH, Young JC (1967) J Chem Soc A 134–137
Arthur NL, Bell TN (1978) Rev Chem Intermed 2:37–74
Kerr JA, Stephens A, Young JC (1969) Int J Chem Kinet 1:371–380
Arthur NL, Christie JR, Mitchell GD (1979) Aust J Chem 32:1017–1023
Bell TN, Johnson BB (1967) Aust J Chem 20:1545–1551
Kerr JA, Slater DH, Young JC (1966) J Chem Soc A 104–108
Bell RL, Truong TN (1994) J Chem Phys 101:10442–10451
Truong TN, Duncan WT, Bell RL (1996) Chemical applications of density functional theory. American Chemical Society, Washington, DC, p 85
Truhlar DG (1995) In: Heidrich D (ed) The reaction path in chemistry:current approaches and perspectives. Kluwer, Dordrecht
Corchado JC, Espinosa-Garcia J, Hu W-P, Rossi I, Truhlar DG (1995) J Phys Chem 99:687–694
Hu W-P, Truhlar DG (1996) J Am Chem Soc 118:860–869
Fast PL, Truhlar DG (2000) J Phys Chem A 104:6111–6116
Corchado JC, Chuang Y-Y, Fast PL, Hu W-P, Liu Y-P, Lynch GC, Nguyen KA, Jackels CF, Fernandez-Ramos A, Ellingson BA, Lynch BJ, Zheng JJ, Melissasa VS, Villa J, Rossi I, Coitino EL, Pu JZ, Albu TV, Steckler R, Garrett BC, Isaacson AD, Truhlar DG (2007) POLYRATE version 9.7. Department of Chemistry and Supercomputer Institute, University of Minnesota, Minneapolis
Truhlar DG, Garrett BC (1980) Acc Chem Res 13:440–448
Truhlar DG, Isaacson AD, Garrett BC (1985) Generalized transtion state theory. In: Baer M (ed) The theory of chemical reaction dynamics, 4th edn. CRC, Boca Raton, p 65
Duncan WT, Truong TN (1995) J Chem Phys 103:9642–9652
Frisch MJ, Head-Gordon M, Pople JA (1990) Chem Phys Lett 166:275–280
Head-Gordon M, Pople JA, Frisch MJ (1988) Chem Phys Lett 153:503–506
Boris B, Petia B (1999) J Phys Chem A 103:6793–6799
Bergman DL, Laaksonen L, Laaksonen A (1997) J Mol Graph Model 15:301–306
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, Li X, Hratchian HP, Izmaylov AF, Bloino J, Zheng G, Sonnenberg JL, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Montgomery JA, Jr, Peralta JE, Ogliaro F, Bearpark M, Heyd JJ, Brothers E, Kudin KN, Staroverov VN, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant JC, Iyengar SS, Tomasi J, Cossi M, Rega N, Millam JM, Klene M, Knox JE, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Martin RL, Morokuma K, Zakrzewski VG, Voth GA, Salvador P, Dannenberg JJ, Dapprich S, Daniels AD, Farkas O, Foresman JB, Ortiz JV, Cioslowski J, Fox DJ (2009) Gaussian, Inc., Revision A.02, Wallingford CT
Garrett BC, Truhlar DG (1979) J Chem Phys 70:1593–1598
Garrett BC, Truhlar DG (1979) J Am Chem Soc 101:4534–4548
Garrett BC, Truhlar DG, Grev RS, Magnuson AW (1980) J Phys Chem 84:1730–1748
Lu DH, Truong TN, Melissas VS, Lynch GC, Liu Y-P, Grarrett BC, Steckler R, Issacson AD, Rai SN, Hancock GC, Lauderdale JG, Joseph T, Truhlar DG (1992) Comput Phys Commun 71:235–262
Liu Y-P, Lynch GC, Truong TN, Lu D-H, Truhlar DG, Garrett BC (1993) J Am Chem Soc 115:2408–2415
Truhlar DG (1991) J Comput Chem 12:266–270
Chuang YY, Truhlar DG (2000) J Chem Phys 112:1221–1228
Kuchitsu K (1998) Structure of free polyatomic molecules basic data. Springer, Berlin, pp 84, 87, 102, 104, 105, 111
Hammond GS (1955) J Am Chem Soc 77:334–338
Jacox ME (2005) In: NIST Chemistry WebBook, NIST Standard Reference Database Number 69, June, Release
Shimanouchi T (2005) In: NIST Chemistry WebBook, NIST Standard Reference Database Number 69, June, Release
Chase MW (1998) NIST-JANAF Themochemical Tables, 4th ed., J Phys Chem Ref Data, Monograph 9, ACS: Washington, DC 1–1951
Afeefy HY, Liebman JF, Stein SE (2005) In: NIST ChemistryWebBook, NIST Standard Reference Database Number 69, June, R
Ho P, Melius CF (1990) J Phys Chem 94:5120–5127
Manion JA (2002) J Phys Chem Ref Data 123–172
Doncaster AM, Walsh R (1978) Int J Chem Kinet 10:101–110
Wu YD, Wong CL (1995) J Org Chem 60:821–828
Walsh R (1989) In: Patai S, Rapport Z (eds) Thermochemistry. In: The chemistry of organo silicon compounds, Part 1, vol Chapter 5. Wiley, New York, pp 371–391
Hildenbrand DL, Lau KH, Sanjurjo A (2003) J Phys Chem A 107:5448–5451
Becerra R, Walsh R (1998) Thermochemistry. In: Rapport Z, Apeloig Y (eds) The chemistry of organic silicon compounds Vol 2, vol Chapter 4. Wiley, New York, pp 153–180
Acknowledgments
The authors thank Professor Donald G. Truhlar for providing the POLYRATE 9.7 program. This work was supported by the National Natural Science Foundation of China (20973077 and 20973049), the Program for New Century Excellent Talents in University (NCET), the Doctoral Fund of Ministry of Education of China (20112303110005), the Foundation for the Department of Education of Heilongjiang Province (1152G010, 11551077), the Key Subject of Science and Technology by the Ministry of Education of China, the SF for leading experts in academe of Harbin of China (2011RFJGS026), the Science Foundation for Distinguished Young Scholar of Heilongjiang Province (JC201206).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Zhang, H., Liu, P., Liu, JY. et al. Theoretical study and rate constant calculations for the reactions of SiHX3 with CF3 and CH3 radicals (X = F, Cl). J Mol Model 19, 1515–1525 (2013). https://doi.org/10.1007/s00894-012-1704-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00894-012-1704-9