Abstract
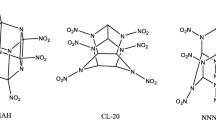
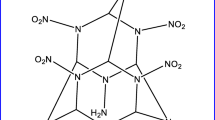
A novel polynitro cage compound 4,8,11,14,15-pentanitro-2,6,9,13-tetraoxa-4,8,11,14,15–pentaazaheptacyclo [5.5.1.13,11.15,9]pentadecane(PNTOPAHP) has been designed and investigated at the DFT-B3LYP/6-31(d) level. Properties, such as electronic structure, IR spectrum, heat of formation, thermodynamic properties and crystal structure have been predicted. This compound is most likely to crystallize in C2/c space group, and the corresponding cell parameters are Z = 8, a = 29.78 Å, b = 6.42 Å, c = 32.69 Å, α = 90.00°, β = 151.05°, γ = 90.00°and ρ = 1.94 g/cm3. In addition, the detonation velocity and pressure have also been calculated by the empirical Kamlet-Jacobs equation. As a result, the detonation velocity and pressure of this compound are 9.82 km/s, 44.67 GPa, respectively, a little higher than those of 4,10-dinitro-2,6,8,12–tetraoxa−4,10-diazaisowurtzitane(TEX, 9.28 km/s, 40.72 GPa). This compound has a comparable chemical stability to TEX, based on the N-NO2 trigger bond length analysis. The bond dissociation energy ranges from 153.09 kJ mol–1 to 186.04 kJ mol–1, which indicates that this compound meets the thermal stability requirement as an exploitable HEDM.





Similar content being viewed by others
References
Klapotke TM (2007) New nitrogen-rich high explosives. Springer, Berlin
Xu XJ, Xiao HM, Ju XH, Gong XD, Zhu WH (2006) Computational studies on polynitrohexazaadmantanes as potential high energy denstiy materials. J Phys Chem A 110:5929–5933
Wang GX, Gong XD, Xiao HM (2009) Theoretical investigation on density, detonation properties, and pyrolysis mechanism of nitro derivatives of benzene and aminobenzenes. Int J Quantum Chem 109:1522–1530
Zhang JY, Du HC, Wang F, Gong XD, Huang YS (2012) Theoretical investigations of a high density cage compound 10-(1-nitro-1,2,3,4-tetraazol-5-yl)methyl-2,4,6,8,12-hexanitrohexazaisowurtzitane. J Mol Model 18:165–170
Zhang JY, Du HC, Wang F, Gong XD, Huang YS (2011) DFT studies on high energy density cage compound 4-trinitroethyl-2,6,8,10,12-pentanitroheazaisowurtzitane. J Phys Chem A 115:6617–6621
Zeman S, Jalovy Z (2000) Heats of fusion of polynitro derivates of polyazaisowurtzitane. Thermochim Acta 345:31–38
Gobel M, Klapotke TM (2009) Development and testing of energetiv materials: the concept of high densities based on the trinitroethyl functionality. Adv Funct Mater 19:347–365
Agrawal JP (2005) Some new high energy materials and their formulations for specialized applications. Propellants Explos Pyrotech 30:316–328
Singh RP, Verma RD, Meshri DT, Shreeve JM (2006) Energetic nitrogen-rich salts and ionic liquids. Angew Chem Int ED 45:3584–3601
Politzer P, Lane P, Murray JS (2011) Computational characterization of a potential energetic compound: 1,3,5,7-tetranitro-2,4,6,8-tetraazacubane. Cent Eur J Energetic Mater 8:39–52
Jalovy Z, Matyas R, Klasovity D, Zeman S (2010) Contribution to the synthesis of 4,10-dinitro 2,6,8,12-tetraoza-4,10-diazatetracyclo[5.5.0.05,903,11]dodecane (TEX). Cent Eur J Chem 7:189–196
Frisch MJ et al. (2003) Gaussian 03, Revision A.1 Gaussian lnc, Pittsburgh, PA
Accelrys (2009) Materials Studio 4.4. Accelrys, San Diego
Becke AD (1993) Density-functional thermochemistry. III. The role of the exact exchange. J Chem Phys 98:5648–5652
Qiu L, Xiao HM, Ju XH, Gong XD (2005) Theoretical study of the structures and properties of cyclic nitramines: tetranitrotetraazadecalin (TNAD) and its isomers. Int J Quantum Chem 105:48–56
Xu XJ, Xiao HM, Gong XD, Ju XH, Chen ZX (2005) Theoretical studies on the vibrational spectra, thermodynamic properties, detonation properties, and pyrolysis mechanisms for polynitroadamantanes. J Phys Chem A 109:11268–11274
Wei T, Zhu WH, Zhang XW, Li YF, Xiao HM (2009) Molecular design of 1,2,4,5-tetrazine-based high-energy density materials. J Phys Chem A 113:9404–9412
Qiu LM, Gong XD, Zheng J, Xiao HM (2009) Theoretical studies on polynitro-1,3-bishomophentaprismanes as potential high energy density compounds. J Hazard Mater 166:931–938
Scott AP, Leo R (1996) Harmonic vibrational frequencies: an evaluation of Hartree−Fock, Møller−Plesset, quadratic configuration interaction, density functional theory, and semiempirical scale factors. J Phys Chem 100:16502–16513
Gutowski KE, Rogers RD, Dixon DA (2007) Accurate thermochemical properties for energetic materials application. II. Heat of formation of imidazolium-, 1,2,4-triazolium-, and tetrazolium-based energetic salts from isodesmic and lattice energy calculations. J Phys Chem B 4788–4800
Guo Y, Gao H, Twamley B, Shreeve JM (2007) Energetic nitrogen rich salts of N, N-bis[1(2)H-tetrazol-5-yl]amine. Adv Mater 19:2884–2888
Fan XW, Ju XH (2008) Theoretical studies on four-membered ring compound with NF2, ONO2, N3, and NO2 group. J Comput Chem 29:505–513
Lide DR (2002) CRC handbook of chemistry and physics. CRC, Boca Raton
Akhavan J (1998) The chemistry of explosive. The Royal Society of Chemistry, Cambridge, UK
Kamlet MJ, Jabcobs SJ (1968) Chemistry of detonation. I. A simple method of calculating the detonation properties of C-H-N-O explosives. J Chem Phys 48:23–35
Li JS (2010) Relationships for the impact sensitivities of energetic C-nitro compounds based on bond dissociation energy. J Phys Chem B 114:2198–2202
Song XS, Cheng XL, Yang XD, Li DH, Feng R, Hu L (2007) Correlation between the bond dissociation energies and impact sensitivities in nitramine and polynitro benzoate molecules with polynitro alkyl groupings. J Hazard Mater 150:317–321
Li XH, Zhang RZ, Zhang XZ (2010) Computational study of imidazole derivative as high energetic materials. J Hazard Mater 183:622–631
Brill BT, James JK (1993) Kinetics and mechanisms of thermal decomposition of nitroaromatic explosives. Chem Rev 93:2667–2692
Gao A, Rheingold AL, Brill BT (2004) Thermal decomposition of energetic materials. A trigger linkage study of high-nitrogen content nitraminotetrazoles and nitramino-1,2,4-triazoles. Propellants Explos Pyrotech 16:97–104
Zhang CY, Shu YJ, Huang YG, Zhao XD, Dong HS (2005) Investigation of correlation between impact sensitivities and nitro group charges in nitro compounds. J Phys Chem B 109:8978–8982
Valencia H, Gil A, Frapper G (2010) Trends in the adsorption of 3d transition metal atoms onto graphene and nanotube surfaces: a DFT study and molecular orbital analysis. J Phys Chem C 114:14141–14153
Shang J, Zhang JG, Zhang TL, Huang HS, Zhang SW, Zhou ZN (2012) First-principles study of energetic complexes (II): (5-cyanotetrazolato-N2)pentaammine cobalt (III) perchlorate (CP) and Ni, Fe and Zn analogues. J Mol Model 18:2855–2860
Wei T, Zhu WH, Zhang JJ, Xiao HM (2010) DFT study on energetic tetrazolo-[1,5-b]-1,2,4,5-tetrazine and 1,2,4-triazolo-[4,3-b]-1,2,4,5-tetrazine derivatives. J Hazard Mater 179:1–3
Hoffmann R (1988) Solids and surfaces: a chemists view of bonding in the extended structures. Wiley-VCH, New York
Wei CX, Huang H, Duan XH, Pei CH (2011) Structures and properties prediction of HMX/TATB co-crystal. Propellants Explos Pyrotech 36:416–423
Liu H, Wang F, Wang GX, Gong XD (2012) Theoretical investigations on structure, density, detonation properties, and sensitivity of the derivatives of PYX. J Comput Chem 33:1790–1796
Li MM, Wang GX, Guo XD, Wu ZW, Song HC (2009) Theoretical studies on the structures, thermodynamic properties, detonation properties, and pyrolysis mechanisms of four trinitrate esters. J Mol Struct THEOCHEM 900:1–3
Chernikova NY, Belskii VK, Zorkii PM (1990) New statistical data on the topology of homomolecular organic crystals. J Struct Chem 31:661–666
Wilson AJC (1988) Space groups rare for organic structures. I. Triclinic, monoclinic and orthorhombic crystal classes. Acta Crystallogr A 44:715–724
Srinivasan R (1992) On space-group frequencies. Acta Crystallogr A 48:917–918
Mighell AD, Himes VL, Rodgers JR (1983) Space group frequencies for organic compounds. Acta Crystallogr A 39:737–740
Baur WH, Kassner D (1992) The perils of Cc: comparing the frequencies of falsely assigned space groups with their general population. Acta Crystallogr B 48:356–369
Xiao HM, Xu XJ, Qiu L (2008) Theoretical design of high energy density materials. Science, Beijing
Acknowledgments
The support of the National Natural Science Foundation of China (Grant No.61106078) and NUST Research Funding (Grant No.2011ZDJH28) are gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lin, H., Zhu, Sg., Zhang, L. et al. Theoretical investigation of a novel high density cage compound 4,8,11,14,15–pentanitro-2,6,9,13–tetraoxa-4,8,11,14,15-pentaazaheptacyclo[5.5.1.13,11. 15,9] pentadecane. J Mol Model 19, 1019–1026 (2013). https://doi.org/10.1007/s00894-012-1629-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00894-012-1629-3