Abstract
Molecular dynamic (MD) simulations have been performed to study the behaviors of ten kinds of cyclo-hexa-peptides (CHPs) composed of amino acids with the diverse hydrophilic/hydrophobic side chains at the water/cyclohexane interface. All the CHPs take the “horse-saddle” conformations at the interface and the hydrophilicity/hydrophobicity of the side chains influences the backbones’ structural deformations. The orientations and distributions of the CHPs at the interface and the differences of interaction energies (ΔΔE) between the CHPs and the two liquid phases have been determined. RDF analysis shows that the H-bonds were formed between the OC atoms of the CHPs’ backbones and Hw atoms of water molecules. N atoms of the CHPs’ backbones formed the H-bonds or van der Waals interactions with the water solvent. It was found that there is a parallel relationship between ΔΔE and the lateral diffusion coefficients (D xy ) of the CHPs at the interface. The movements of water molecules close to the interface are confined to some extent, indicating that the dynamics of the CHPs and interfacial water molecules are strongly coupled.

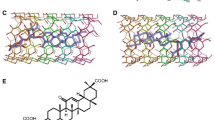
Scheme of the ten kinds of CHPs formed by even alternating D- and L- amino acids with the different hydrophilic/hydrophobic side chains. The letters in the parentheses stand for the abbreviations of the composed amino acids in the CHPs











Similar content being viewed by others
References
Vollenbroich D, Ozel M, Vater J, Kamp RM, Pauli G (1997) Mechanism of inactivation of enveloped viruses by the biosurfactant surfactin from bacillus subtilis. Biologicals 25:289–297
Kracht M, Rokos H, Ozel M, Kowall M, Pauli G, Vater J (1999) Antiviral and hemolytic activities of surfactin isoforms and their methyl ester derivative. J Antibiot 52:613–619
Tendulkar SR, Saikumari YK, Patel V, Raghotama S, Munshi TK, Balaram P, Chattoo BB (2007) Isolation, purification and characterization of an antifungal molecule produced by bacillus licheniformis BC98, and its effect on phytopathogen magnaporthe grise. J Appl Microbiol 103:2331–2339
Weber C, Wider G, von Freyberg B, Traber R, Braun W, Widmer H, Wuthrich K (1991) The NMR structure of cyclosporin a bound to cyclophilin in aqueous solution. Biochemistry 30:6563–6574
Trevisan G, Maldaner G, Velloso NA, Sant’Anna GS, Ilha V, Gewehr CCV, Rubin MA, Morel AF, Ferreira J (2009) Antinociceptive effects of 14-membered cyclopeptide alkaloids. J Nat Prod 72:608–612
Gang HZ, Liu JF, Mu BZ (2010) Molecular dynamics simulation of Surfactin derivatives at the decane/water interface at Low surface coverage. J Phys Chem B 114:2728–2737
Liu J, Fan JF, Tang M, Zhou WQ (2010) Molecular dynamics simulation for the structure of the water chain in a transmembrane peptide nanotube. J Phys Chem A 114:2376–2383
Bagheri M, Keller S, Dathe M (2011) Interaction of W-substituted analogs of cyclo- RRRWFW with bacterial lipopolysaccharides: the role of the aromatic cluster in antimicrobial activity. Antimicrob Agents Chemother 55:788–797
Jelokhani-Niaraki M, Kondejewski LH, Wheaton LC, Hodges RS (2009) Effect of ring size on conformation and biological activity of cyclic cationic antimicrobial peptides. J Med Chem 52:2090–2097
Praveena G, Kolandaivel P (2008) Structural and dynamical studies of all-trans and all-cis Cyclo[(1R,3S)-γ-Acc-Gly]3 peptides. J Mol Model 14:1147–1157
Praveena G, Kolandaivel P (2009) Interaction of metal ions with Cyclo[(1R,3S)-c-Acc-Gly]3 Hexapeptide –a theoretical study. J Mol Struct (THEOCHEM) 900:96–102
Leodidis EB, Hatton TA (1990) Amino acids in AOT reversed Micelles. 2. The hydrophobic effect and hydrogen bonding as driving forces for interfacial solubilization. J Phys Chem 94:6411–6420
Wolfenden R, Andersson L, Cullis PM, Southgate CCB (1981) Affinities of amino acid side chains for solvent water. Biochemistry 20:849–855
Horinek D, Netz RR (2011) Can simulations quantitatively predict peptide transfer free energies to urea solutions? thermodynamic concepts and force field limitations. J Phys Chem A 115:6125–6136
Nicolas JP (2003) Molecular dynamics simulation of surfactin molecules at the water- hexane interface. Biophys J 85:1377–1391
Bonmatin JM, Genest M, Labbe H, Ptak M (1994) Solution three-dimensional structure of surfactin: a cyclic lipopeptide studied by 1 H-NMR, distance geometry, and molecular dynamics. Biopolymers 34:975–986
Gallet X, Deleu M, Razafindralambo H et al (1999) Computer simulation of surfactin conformation at a hydrophobic/hydrophilic interface. Langmuir 15:2409–2413
Gang HZ, Liu JF, Mu BZ (2010) Interfacial behavior of surfactin at the decane/water interface: a molecular dynamics simulation. J Phys Chem B 114(46):14947–14954
Ahmad F, Constabel F, Geckler KE, Seeck OH, Seo YS et al (2005) X-ray reflectivity study of cyclic peptide monolayers at the air–water interface. Israel J Chem 45:345–352
Ulmschneider JP, Smith JC, White SH, Ulmschneider MB (2011) In silico partitioning and transmembrane insertion of hydrophobic peptides under equilibrium conditions. J Am Chem Soc 133:15487–15495
Khurana E, DeVane RH, Kohlmeyer A, Klein ML (2008) Probing peptide nanotube self-assembly at a liquid-liquid interface with coarse-grained molecular dynamics. Nano Lett 8:3626–3630
Rapaport H, Kim HS, Kiaer K et al (1999) Crystalline cyclic peptide nanotubes at interfaces. J Am Chem Soc 121:1186–1191
Ghadiri MR, Granja JR, Milligan RA, Duncan EM, Khazanovich N (1993) Self-assembling organic nanotubes based on a cyclic peptide architecture. Nature 366:324–327
Liu J, Fan JF, Tang M, Cen M, Yan JF et al (2010) Water diffusion behaviors and transportation properties in transmembrane cyclic hexa-, octa- and decapeptide nanotubes. J Phys Chem B 114:12183–12192
Sun H (1998) COMPASS: an ab initio force-field optimized for condensed-phase applications-overview with details on alkane and benzene compounds. J Phys Chem B 102:7338–7364
McCool MA, Woolf LA (1972) Self-diffusion measurements under pressure with a diaphragm cell. Theory of the method and experimental results for cyclohexane. High Temp-High Press 4:85–95
Nose S (1984) A molecular dynamics method for simulations in the Canonocal ensemble. Mol Phys 52:255–268
Berendsen HJC, Postma JPM, van Gunsteren WE et al (1984) Molecular dynamics with coupling to an external bath. J Chem Phys 81:3684–3692
Allen MP, Tildesley DJ (1989) Computer simulation of liquids. Clarendon, Oxford
Smith W (1992) A replicated data molecular dynamics strategy for the parallel Ewald Sum. Comput Phys Commun 67:392–406
Pang JY, Wang YJ, Xu GY, Han TT (2011) Molecular dynamics simulations of SDS, DTAB, and C12E8 monolayers adsorbed at the air/water surface in the presence of DSEP. J Phys Chem B 115:2518–2526
Sedlmeier F, von Hansen Y, Mengyu L, Horinek D, Netz RR (2011) Water dynamics at interfaces and solutes: disentangling free energy and diffusivity contributions. J Stat Phys 145:240–252
Jang SS, Goddard WA (2006) Structures and properties of Newton black films characterized using molecular dynamics simulations. J Phys Chem B 110:7992–8001
Mark P, Nilsson L (2001) Structure and dynamics of the TIP3P, SPC, and SPC/E water models at 298 K. J Phys Chem A 105:9954–9960
Acknowledgments
This work has been supported by the National Natural Science Foundation of China (Grant No. 21173154) and the Priority Academic Program Development of Jiangsu Higher Education Institutions. The authors are grateful to College of Computer Science & Technology in Soochow University for providing amounts of computer facilities assignment on its high-powered computers.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cen, M., Fan, J.F., Liu, D.Y. et al. Cyclo-hexa-peptides at the water/cyclohexane interface: a molecular dynamics simulation. J Mol Model 19, 601–611 (2013). https://doi.org/10.1007/s00894-012-1588-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00894-012-1588-8