Abstract
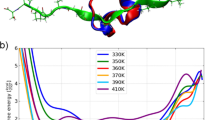
Geometry optimization results are reported for secondary structural elements of small proteins and polypeptides. Emphasis is placed on how well molecular mechanics as well as semiempirical, ab initio, and density functional methods describe α-helical and related structures in purely theoretical models (Gly10, Ile10) as well as in realistic models (an α-helical region of calmodulin, and the complete structure of a small protein). Many of the methods examined here were found to provide unsatisfactory descriptions of the hydrogen-bonding interactions within polypeptide-type structures, as the α-helical canonical secondary structure motif was not reproduced accurately. Ab initio and DFT methods provided reasonable results only when solvation models were included, although Hartree–Fock failed even with solvation in one of the test cases; among the semiempirical methods, one of the PM6 implementations performed very well.






Similar content being viewed by others
References
Noodleman L, Lovell T, Han WG, Li J, Himo F (2004) Quantum chemical studies of intermediates and reaction pathways in selected enzymes and catalytic synthetic systems. Chem Rev 104:459–508
Siegbahn PE, Blomberg MRA (2000) Transition-metal systems in biochemistry studied by high-accuracy quantum chemical methods. Chem Rev 100:421–437
Harris DL (2001) High-valent intermediates of heme proteins and model compounds. Curr Opin Chem Biol 5:724–735
Friesner RA, Dunietz BD (2001) Large-scale ab initio quantum chemical calculations on biological systems. Acc Chem Res 34:351–358
Gooding SR, Winn PJ, Jones GA, Ferenczy GG, Frusher MJ, Reynolds CA (2006) Classical polarization in hybrid QM/MM methods. J Phys Chem A 110:6487–6497
Warshel A, Parson WW (2001) Dynamics of biochemical and biophysical reactions: insight from computer simulations. Q Rev Biophys 34:563–679
Warshel A (2002) Molecular dynamics simulations of biological reactions. Acc Chem Res 35:385–395
Rosta E, Klahn M, Warshel A (2006) Towards accurate ab initio QM/MM calculations of free-energy profiles of enzymatic reactions. J Phys Chem B 110:2934–2941
Ryde U (2003) Combined quantum and molecular mechanics calculations on metalloproteins. Curr Opin Chem Biol 7:136–142
Silaghi-Dumitrescu R (2004) On the performance of the PM3 semiempirical method with heme complexes relevant to dioxygen and peroxide activation. Rev Chim 55:304–307
Tejero I, Gonzalez-Lafont A, Lluch JM (2007) A PM3/d specific reaction parameterization for iron atom in the hydrogen abstraction catalyzed by soybean lipoxygenase-1. J Comput Chem 28:997–1005
Mcnamara JP, Sundararajan M, Hillier IH, Ge J, Campbell A, Morgado C (2006) Can the semiempirical PM3 scheme describe iron-containing bioinorganic molecules? J Comput Chem 27:1307–1323
Mcnamara JP, Sundararajan M, Hillier IH (2005) Development of parameter sets for semi-empirical MO calculations of transition metal systems: iron parameters for iron–sulfur proteins. J Mol Graph Model 24:128–137
Csontos J, Palermo NY, Murphy RF, Lovas S (2008) Calculation of weakly polar interaction energies in polypeptides using density functional and local Moller–Plesset perturbation theory. J Comput Chem 29:1344–1352
Sulpizi M, Raugei S, Vandevondele J, Carloni P, Sprik M (2007) Calculation of redox properties: understanding short- and long-range effects in rubredoxin. J Phys Chem B 111:3969–3976
Jensen KP (2006) Iron–sulfur clusters: why iron? J Inorg Biochem 100:1436–1439
Sundararajan M, Hillier IH, Burton NA (2006) Structure and redox properties of the protein, rubredoxin, and its ligand and metal mutants studied by electronic structure calculation. J Phys Chem A 110:785–790
Machonkin TE, Westler WM, Markley JL (2005) Paramagnetic NMR spectroscopy and density functional calculations in the analysis of the geometric and electronic structures of iron–sulfur proteins. Inorg Chem 44:779–797
Vrajmasu VV, Munck E, Bominaar EL (2004) Theoretical analysis of the three-dimensional structure of tetrathiolato iron complexes. Inorg Chem 43:4867–4879
Kennepohl P, Solomon EI (2003) Electronic structure contributions to electron-transfer reactivity in iron–sulfur active sites: 3. Kinetics of electron transfer. Inorg Chem 42:696–708
Vrajmasu VV, Bominaar EL, Meyer J, Munck E (2002) Mossbauer study of reduced rubredoxin as purified and in whole cells. Structural correlation analysis of spin Hamiltonian parameters. Inorg Chem 41:6358–6371
Glaser T, Rose K, Shadle SE, Hedman B, Hodgson KO, Solomon EI (2001) S K-edge X-ray absorption studies of tetranuclear iron–sulfur clusters: mu-sulfide bonding and its contribution to electron delocalization. J Am Chem Soc 123:442–454
Sigfridsson E, Olsson MH, Ryde U (2001) Inner-sphere reorganization energy of iron–sulfur clusters studied with theoretical methods. Inorg Chem 40:2509–2519
Kloiber K, Weiskirchen R, Krautler B, Bister K, Konrat R (1999) Mutational analysis and NMR spectroscopy of quail cysteine and glycine-rich protein CRP2 reveal an intrinsic segmental flexibility of LIM domains. J Mol Biol 292:893–908
Nayal M, Di Cera E (1996) Valence screening of water in protein crystals reveals potential Na+ binding sites. J Mol Biol 256:228–234
Rossi M, Blum V, Kupser P, Helden V, Bierau F, Pagel K, Meijer G, Scheffler M (2010) Secondary structure of Ac-Ala n -LysH+ polyalanine peptides (n = 5, 10, 15) in vacuo: helical or not? J Phys Chem Lett 1:3465–3470
Hua S, Xu L, Li S (2011) Cooperativity in long α- and 310-helical polyalanines: both electrostatic and van der Waals interactions are essential. J Phys Chem B 115:11462–11469
Tkatchenko A, Rossi M, Blum V, Ireta J, Scheffler M (2011) Unraveling the stability of polypeptide helices: critical role of van der Waals interactions. Phys Rev Lett 106:118102
Wavefunction, Inc. (1998)Spartan 5.0, Wavefunction, Inc., Irvine
Frisch MJ, Trucks GW, H. B. Schlegel HB, Scuseria GE, Robb, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, Li X, Hratchian HP, Izmaylov AF, Bloino J, Zheng G, Sonnenberg JL, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Montgomery, Jr JA, Peralta JE, Ogliaro F, Bearpark M, Heyd JJ, Brothers E, Kudin KN, Staroverov VN, Kobayashi R, Normand J, Raghavachari K, Rendell K, Burant JC, Iyengar SS, Tomasi J, Cossi M, Rega N, Millam JM, Klene M, Knox JE, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Martin RL, Morokuma K, Zakrzewski VG, Voth GA, Salvador P, Dannenberg JJ, Dapprich S, Daniels AD, Farkas O, Foresman JB, Ortiz JV, Cioslowski J, Fox DJ (2009) Gaussian 09, revision A.02. Gaussian Inc., Wallingford
Hypercube, Inc. (2007) HyperChem molecular modelling system, release 8.0 for Windows. Hypercube, Inc.
Stewart JJP (2009) MOPAC2009, version 10.153L. Stewart Computational Chemistry, Colorado Springs
Eckert F, Klamt A (2002) COSMO solvation model. AICHE J 48:369–385
Kudin KN, Scuseria GE, Cancès E (2002) A black-box self-consistent field convergence algorithm: one step closer. J Chem Phys 116:8255–8261
Kun ZA, Lupan A, Silaghi-Dumitrescu R (2010) PM6 modeling of alpha helical polypeptide structures. Studia Universitatis Babes-Bolyai Chemia 55:31–36
Korth M, Pitonak M, Rezac J, Hobza P (2010) A transferable H-bonding correction for semiempirical quantum-chemical methods. J Chem Theor Comput 6:344–352
Klamt A, Schüürmann G (1993) COSMO: a new approach to dielectric screening in solvents with explicit expressions for the screening energy and its gradient. J Chem Soc Perkin Trans 2:799–805
Baker J (1986) An algorithm for the location of transition states. J Comput Chem 7:385–395
Davidon WC (1991) Variable metric method for minimization. SIAM J Optim 1:1–17
Fletcher R (1987) Practical methods of optimization, 2nd edn. Wiley, New York
Broyden CG (1970) The convergence of a class of double-rank minimization algorithms. II. The new algorithm. J Inst Math Appl 6:222–231
Fletcher R (1970) A new approach to variable metric algorithms. Comput J 13:317–322
Goldfarb D (1970) A family of variable-metric methods derived by variational means. Math Comput 24:23–26
Shanno DF (1970) Conditioning of quasi-Newton methods for function minimization. Math Comput 24:647–656
Matthies H, Strang G (1979) The solution of nonlinear finite element equations. Int J Num Meth Eng 14:1613–1626
Nocedal J (1980) Updating quasi-Newton matrices with limited storage. Math Comput 35:773–782
Byrd RH, Nocedal J, Schnabel RB (1994) Representations of quasi-Newton matrices and their use in limited memory methods. Math Prog 63:129–156
Babu YS, Bugg CE, Cook WJ (1988) Structure of calmodulin refined at 2.2 Å resolution. J Mol Biol 204:191–204
Nordhoff A, Tziatzios C, Van Den Broek JA, Schott MK, Kalbitzer HR, Becker K, Schubert D, Schirmer RH (1997) Solution structure of an HGR inhibitor. Eur J Biochem 245:273–282
Acknowledgments
Funding from the Romanian Ministry of Education and Research (grants PN II 312/2008 and Parteneriate 72168/2008-FLUORODENT) is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOC 4124 kb)
Rights and permissions
About this article
Cite this article
Lupan, A., Kun, AZ., Carrascoza, F. et al. Performance comparison of computational methods for modeling alpha-helical structures. J Mol Model 19, 193–203 (2013). https://doi.org/10.1007/s00894-012-1531-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00894-012-1531-z