Abstract
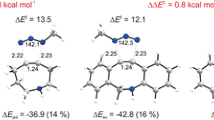
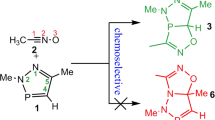
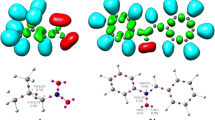
The mechanisms of cycloaddition reactions between 1-aza-2-azoniaallene cations 1 and acetylenes 2 have been investigated using the global electrophilicity and nucleophilicity of the corresponding reactants as global reactivity indexes defined within the conceptual density functional theory. The reactivity and regioselectivity of these reactions were predicted by analysis of the energies, geometries, and electronic nature of the transition state structures. The theoretical results revealed that the reaction features a tandem process: an ionic 1,3-dipolar cycloaddition to produce the cycloadducts 3 H-pyrazolium salts 3 followed by a [1,2]-shift affording the thermodynamically more stable adducts 4 or 5. The mechanism of the cycloaddition reactions can be described as an asynchronous concerted pathway with reverse electron demand. The model reaction has also been investigated at the QCISD/6-31++G(d,p) and CCSD(T)/6-31++G(d,p)//B3LYP/6-31++G(d,p) levels as well as by the DFT. The polarizable continuum model, at the B3LYP/6-31++G(d,p) level of theory, was used to study solvent effects on all the studied reactions. In solvent dichloromethane, all the initial cycloadducts 3 were obtained via direct ionic process as the result of the solvent effect. The consecutive [1,2]-shift reaction, in which intermediates 3 are rearranged to the five-membered heterocycles 4/5, is proved to be a kinetically controlled reaction, and the regioselectivity can be modulated by varying the migrant. The LOL function and RDG function based on localized electron analysis were used to analysis the covalent bond and noncovalent interactions in order to unravel the mechanism of the title reactions.








Similar content being viewed by others
Abbreviations
- B3LYP:
-
Becke-3-parameter-Lee-Yang-Parr
- QCISD:
-
Quadratic configuration interaction using single and double substitutions
- CCSD(T):
-
Coupled cluster calculations with single and double excitations and a perturbative estimate of triple contributions
- DFT:
-
Density functional theory
- PCM:
-
Polarized continuum model
- IRC:
-
Intrinsic reaction coordinate
- NBO:
-
Natural bond orbital
- LOL:
-
Localized orbital locator
- RDG:
-
Reduced density gradient
- AM1:
-
Austin model 1
References
Huisgen R (1963) 1,3-Dipolar cycloadditions. Past and future. Angew Chem Int Edit 2(10):565–598
Padwa A, Pearson WH (2002) Synthetic applications of 1,3 dipolar cycloaddition chemistry toward heterocycles and natural products. Wiley, New York
Moura NMM, Giuntini F, Faustino MAF, Neves M, Tome AC, Silva AMS, Rakib EM, Hannioui A, Abouricha S, Roder B, Cavaleiro JAS (2010) 1,3-Dipolar cycloaddition of nitrile imines to meso-tetraarylporphyrins. ARKIVOC (Part 5):24-33
Song Z, He XP, Jin XP, Gao LX, Sheng L, Zhou YB, Li J, Chen GR (2011) ‘Click’ to bidentate bis-triazolyl sugar derivatives with promising biological and optical features. Tetrahedron Lett 52(8):894–898
Cases M, Duran M, Sole M (2000) The [2 + 1] cycloaddition of singlet oxycarbonylnitrenes to C 60. J Mol Model 6(2):205–212. doi:10.1007/s0089400060205
Kuznetsov ML, Kukushkin VY, Dement’Ev AI, Pombeiro AJL (2003) 1,3-dipolar cycloaddition of nitrones to free and Pt-bound nitriles. A theoretical study of the activation effect, reactivity, and mechanism. J Phys Chem A 107(31):6108–6120
Meng LP, Wang SC, Fettinger JC, Kurth MJ, Tantillo DJ (2009) Controlling selectivity for cycloadditions of nitrones and alkenes tethered by benzimidazoles: combining experiment and theory G-3263-2010. Eur J Org Chem 2009(10):1578–1584
Saeed A, Al-Masoudi NA, Ahmed AA, Pannecouque C (2011) New substituted thiazol-2-ylidene-benzamides and their reaction with 1-Aza-2-azoniaallene salts. Synthesis and anti-HIV activity. Z Naturforsch 66(5):512–520
Weingarten MD, Prein M, Price AT, Snyder JP, Padwa A (1997) Theoretical insights regarding the cycloaddition behavior of push-pull stabilized carbonyl ylides. J Org Chem 62(7):2001–2010
Kuznetsov ML (2006) Theoretical studies of [3 + 2]-cycloaddition reactions. USPEKHI KHIMII 75(11):1045–1073
Gaich T, Baran PS (2010) Aiming for the ideal synthesis. J Org Chem 75(14):4657–4673
Meng Q, Li M (2012) Theoretical studies on the Mo-catalyzed asymmetric intramolecular Pauson-Khand-type [2 + 2 + 1] cycloadditions of 3-allyloxy-1-propynylphosphonates. J Mol Model doi:10.1007/s00894-012-1361-z
Houk KN, Firestone RA, Munchausen LL, Mueller PH, Arison BH, Garcia LA (1985) stereospecificity of 1,3-dipolar cyclo-additions of para-nitrobenzonitrile oxide to cis-dideuterioethylene and trans-dideuterioethylene. J Am Chem Soc 107(24):7227–7228
Di Valentin C, Freccero M, Gandolfi R, Rastelli A (2000) Concerted vs stepwise mechanism in 1,3-dipolar cycloaddition of nitrone to ethene, cyclobutadiene, and benzocyclobutadiene: a computational study. J Org Chem 65(19):6112–6120
Ponti A, Molteni G (2001) DFT-based quantitative prediction of regioselectivity: cycloaddition of nitrilimines to methyl propiolate. J Org Chem 66(15):5252–5255
Wang QR, Jochims JC, Kohlbrandt S, Dahlenburg L, Altalib M, Hamed A, Ismail AEH (1992) 1,2,4-triazolium salts from the reaction of 1-aza-2-azoniaallene salts with nitriles. Synth Stuttgart 24(7):710–718
Wirschun W, Winkler M, Lutz K, Jochims JC (1998) 1,3-diaza-2-azoniaallene salts: cycloadditions to alkynes, carbodiimides and cyanamides. J Chem Soc Perk T 1(11):1755–1761
Li ZM, Wang QR (2008) A theoretical investigation on the cycloaddition reaction between azocarbenium ions and nitriles. Int J Quantum Chem 108(6):1067–1075
Wirschun W, Jochims JC (1997) 1,3-diaza-2-azoniaallene salts, novel N-3-building blocks: Preparation and cycloadditions to olefins. Synth Stuttgart 29(2):233–241
Wei MJ, Fang DC, Liu RZ (2002) Theoretical studies on cycloaddition reactions between 1-aza-2-azoniaallene cation and olefins. J Org Chem 67(21):7432–7438
Abuelhalawa R, Jochims JC (1992) on the reaction of n-alkylnitrilium salts with acetylenes - a new synthesis of 2-azoniaallene salts. Synth Stuttgart 24(9):871–874
Wang QR, Altalib M, Jochims JC (1994) On the reaction of 1-aza-2-azoniaallene salts with acetylenes. Chem Ber 127(3):541–547
Wang QR, Amer A, Troll C, Fischer H, Jochims JC (1993) On the reaction of 1-aza-2-azoniaallene salts with carbodiimides. Chem Ber 126(11):2519–2524
Wang JM, Li ZM, Wang QR, Tao FG (2012) A DFT study on the mechanisms for the cycloaddition reactions between 1-Aza-2-azoniaallene cations and carbodiimides. Int J Quantum Chem 112(3):809–822. doi:10.1002/qua.23065
Wang QR, Mohr S, Jochims JC (1994) On the reaction of 1-aza-2-azoniaallene salts with isocyanates. Chem Ber 127(5):947–953
Wei MJ, Fang DC, Liu RZ (2004) Theoretical studies on cycloaddition reactions between 1-aza-2-azoniaallene cations and isocyanates. Eur J Org Chem 2004(19):4070–4076
Wyman J, Javed MI, Al-Bataineh N, Brewer M (2010) Synthetic approaches to bicyclic diazenium salts. J Org Chem 75(23):8078–8087
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, Li X, Hratchian HP, Izmaylov AF, Bloino J, Zheng G, Sonnenberg JL, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Montgomery JA Jr, Peralta JE, Ogliaro F, Bearpark M, Heyd JJ, Brothers E, Kudin KN, Staroverov VN, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant JC, Iyengar SS, Tomasi J, Cossi M, Rega N, Millam NJ, Klene M, Knox JE, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Martin RL, Morokuma K, Zakrzewski VG, Voth GA, Salvador P, Dannenberg JJ, Dapprich S, Daniels AD, Farkas, Foresman JB, Ortiz JV, Cioslowski J, Fox DJ (2009) Gaussian 09, A.02nd edn. Gaussian Inc, Wallingford
Gonzalez C, Schlegel HB (1989) An improved algorithm for reaction-path following. J Chem Phys 90(4):2154–2161
Gonzalez C, Schlegel HB (1990) Reaction-path following in mass-weighted internal coordinates. J Org Chem 94(14):5523–5527
Gauss J, Cremer D (1988) Analytical evaluation of energy gradients in quadratic configuration interaction theory. Chem Phys Lett 150(3–4):280–286. doi:10.1016/0009-2614(88)80042-3
Salter EA, Trucks GW, Bartlett RJ (1989) Analytic energy derivatives in many-body methods. I. First derivatives. J Chem Phys 90(3):1752–1766
Watts JD, Gauss J, Bartlett RJ (1992) Open-shell analytical energy gradients for triple excitation many-body, coupled-cluster methods: MBPT(4), CCSD + T(CCSD), CCSD(T), and QCISD(T). Chem Phys Lett 200(1–2):1–7. doi:10.1016/0009-2614(92)87036-o
Scuseria GE (1991) Analytic evaluation of energy gradients for the singles and doubles coupled cluster method including perturbative triple excitations: theory and applications to FOOF and Cr[sub 2]. J Chem Phys 94(1):442–447
Miertus S, Scrocco E, Tomasi J (1981) Electrostatic interaction of a solute with a continuum - a direct utilization of abinitio molecular potentials for the prevision of solvent effects. Chem Phys 55(1):117–129
Barone V, Cossi M, Tomasi J (1997) A new definition of cavities for the computation of solvation free energies by the polarizable continuum model. J Chem Phys 107(8):3210–3221
Cances E, Mennucci B, Tomasi J (1997) A new integral equation formalism for the polarizable continuum model: theoretical background and applications to isotropic and anisotropic dielectrics. J Chem Phys 107(8):3032–3041
Cossi M, Barone V, Mennucci B, Tomasi J (1998) Ab initio study of ionic solutions by a polarizable continuum dielectric model. Chem Phys Lett 286(3–4):253–260
Perez P (2004) Relationship between superelectrophilicity and the electrophilicity index of isolated species. J Org Chem 69(15):5048–5053. doi:10.1021/jo049945f
Noorizadeh S, Shakerzadeh E (2008) A new scale of electronegativity based on electrophilicity index. J Phys Chem A 112(15):3486–3491. doi:10.1021/jp709877h
Chattaraj PK, Giri S, Duley S (2011) Update 2 of: electrophilicity index. Chem Rev 111(2):PR43–PR75. doi:10.1021/cr100149p
Chattaraj PK, Duley S, Domingo LR (2012) Understanding local electrophilicity/nucleophilicity activation through a single reactivity difference index. Org Biomol Chem 10(14):2855–2861. doi:10.1039/c2ob06943a
Domingo LR, Chamorro E, Perez P (2008) Understanding the reactivity of captodative ethylenes in polar cycloaddition reactions: a theoretical study. J Org Chem 73(12):4615–4624. doi:10.1021/jo800572a
Glendening ED, Badenhoop JK, Reed AE, Carpenter JE, Bohmann JA, Morales CM, Weinhold F, Morales M, Weinhold F (2010) NBO 5.9. Theoretical Chemistry Institute, University of Wisconsin, Madison
Yin B, Huang Y, Wang G, Wang Y (2010) Combined DFT and NBO study on the electronic basis of Si···N-β-donor bond. J Mol Model 16(3):437–446. doi:10.1007/s00894-009-0560-8
Davari M, Bahrami H, Haghighi Z, Zahedi M (2010) Quantum chemical investigation of intramolecular thione-thiol tautomerism of 1,2,4-triazole-3-thione and its disubstituted derivatives. J Mol Model 16(5):841–855. doi:10.1007/s00894-009-0585-z
Jacobsen H (2008) Localized-orbital locator (LOL) profiles of chemical bonding. Can J Chem 86(7):695–702
Jacobsen H (2009) Chemical bonding in view of electron charge density and kinetic energy density descriptors. J Comput Chem 30(7):1093–1102. doi:10.1002/jcc.21135
Yang Y (2010) Hexacoordinate bonding and aromaticity in silicon phthalocyanine. J Phys Chem A 114(50):13257–13267. doi:10.1021/jp109278v
Steinmann SN, Mo Y, Corminboeuf C (2011) How do electron localization functions describe pi-electron delocalization? Phys Chem Chem Phys 13(46):20584–20592. doi:10.1039/c1cp21055f
Johnson ER, Keinan S, Mori-Sanchez P, Contreras-Garcia J, Cohen AJ, Yang W (2010) Revealing noncovalent interactions. J Am Chem Soc 132(18):6498–6506. doi:10.1021/ja100936w
Lu T (2011) Multiwfn 2.21. School of Chemical and Biological Engineering. University of Science and Technology Beijing, China
Reed AE, Curtiss LA, Weinhold F (1988) Intermolecular interactions from a natural bond orbital, donor-acceptor viewpoint. Chem Rev 88(6):899–926. doi:10.1021/cr00088a005
Sustmann R (1971) A simple model for substituent effects in cycloaddition reactions. II. The diels-alder reaction. Tetrahedron Lett 12(29):2721–2724. doi:10.1016/s0040-4039(01)96962-x
Acknowledgments
We acknowledge the assistance of other members of Professor Quan-rui Wang's group for obtaining experimental information. This work was supported by the National Natural Science Foundation of China (No. 21102019) and the Science Fund for Distinguished Young Scholars at Fudan University.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, Jm., Li, Zm., Wang, Qr. et al. A DFT study on the mechanisms for the cycloaddition reactions between 1−aza-2-azoniaallene cations and acetylenes. J Mol Model 19, 83–95 (2013). https://doi.org/10.1007/s00894-012-1521-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00894-012-1521-1