Abstract
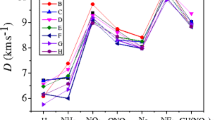
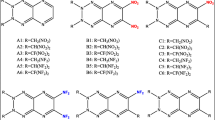
We have studied herein the effect of position and the number of -NO, -NO2, -NH2 and -CH3 groups on the structure, stability, impact sensitivity, density, thermodynamic and detonation properties of triazolones by performing density functional theory calculations at the B3LYP/aug-cc-pVDZ level. The optimized structures, vibrational frequencies and thermodynamic values for triazolones have been obtained in their ground state. Kamlet-Jacob equations were used to calculate the detonation velocity and detonation pressure of model compounds. The detonation properties of NNTO (D 8.75 to 9.10 km/s, P 34.0 to 37.57 GPa), DNTO (D 8.80 to 9.05 km/s, P 35.55 to 38.27 GPa), ADNTO (D 9.01 to 9.42 km/s and P 37.81 to 41.10 GPa) and ANNTO (D 8.58 to 9.0 km/s, P 30.81 to 36.25 GPa) are compared with those of 1,3,5-trinitro-1,3,5-triazine (RDX) (D 8.75 km/s, P 34.70 Gpa) and octahydro-1,3,5,7-tetranitro-1,3,5,7-tetrazocine (HMX) (D 8.96 km/s, P 35.96 GPa). The designed compounds satisfy the criteria of high energy materials.



Similar content being viewed by others
References
Larina L, Lopyrev L (2009) Nitroazoles: synthesis, structure and applications. Springer, New York
Lee KY, Stinecipher Mn (1989) Prop Explos Pyretech 14:241–244
Lee KY, Chapman LB, Coburn MDv (1987) J Energ Mater 5:27–33
Ritchie JP (1989) J Org Chem 54:3553–3560
Williams GK, Brill TBv (1995) J Phys Chem 99:12536–12539
Manchot VW, Noll R (1905) J Liebigs Ann der Chem 343:1–27
Lee KY, Coburn MD (1985) 3-Nitro-1,2,4-triazol-5-one, a less sensitive explosive (LA10302- MS). Los Alamos National Laboratory, Los Alamos
Smith MW, Cliff MD (1999) NTO based explosive formulations: a technology review (DSTO-TR-0796), MRL Technical Report, AR-1-873. Material Research Laboratory, Maribyrnong
Lee KY, Stinecipher MM (1983) United States Patent 5:256,792
Haixia M, Jirong S, Xiaohong S, Rongzu H, Shengli G, Kaibei Y (2002) Thermochim Acta 389:43–47
Cromer DT, Hall JH, Lee KY, Ryan RR (1988) Acta Cryst 44:1144–1147
Cromer DT, Hall JH, Lee KY, Ryan RR (1988) Acta Cryst 44:2206–2208
Hiskey MA, Stinecipher MM, Brown JE (1993) J Energ Mater 11:157–165
Yi X, Rongzu H, Chaoqing Y, Guofu F, Jihua Z (1992) Prop Explos Pyretech 17:298–302
Jiarong L, Boren C, Yuxiang O, Neijue Z (1991) Prop Explos Pyretech 16:145–146
Cheng Wei C, Yeong-Ming W, Te-Chuan C, Cheng C (1997) Prop Explos Pyretech 22:240–241
Redman LD, Spear RJ (1989) An evaluation of metal Salts of 3-nitro-1,2,4-triazol-5-one (NTO) as potential primary explosives, MRL-TN-563. Materials Research Laboratory, Melbourne
Owens FJ (1996) J Mol Struct (Theochem) 370:11–16
Kohno Y, Takahashi O, Saito K (2001) Phys Chem Chem Phys 3:2742–2746
Hiyoshi RI, Kohno Y, Nakamura J (2004) J Phys Chem A 108:5915–5920
Ma HM, Song JR, Dong W, Hu RZ, Zhai GH, Wen ZY (2004) J Mol Struct (Theochem) 678:217–222
Singh G, Felix SP (2003) J Mol Struct 649:71–83
Harris NJ, Lamnestsma K (1996) J Am Chem Soc 118:8048–8055
Turker L, Atalar T (2006) J Hazar Mater A 137:1333–1344
Turker L, Bayer C (2012) J Energ Mater 30:72–96
Klene M, Li X, Knox J, Hratchian HP, Cross JB, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Ayala PY, Morokuma K, Voth GA, Salvador P, Dannenberg JJ, Zakrzewski VG, Dapprich S, Daniels AD, Strain MC, Farkas O, Malick DK, Rabuck AD, Raghavachari K, Foresman JB, Ortiz JV, Cui Q, Baboul AG, Clifford S, Cioslowski J, Stefanov BB, Liu G, Liashenko A, Piskorz P, Komaromi I, Martin RL, Fox DJ, Keith T, Al-Laham MA, Peng CY, Nanayakkara A, Challacombe M, Gill PMW, Johnson B, Chen W, Wong MW, Gonzalez C, Pople JA (2003) Gaussian 03, revision B.04. Gaussian Inc, Pittsburgh
Kohn W, Sham LJ (1965) Phys Rev 140:A1133–A1138
Parr RG, Yang W (1989) Density Functional Theory of Atoms and Molecules. Oxford University Press, London
Becke AD (1988) Phys Rev A 38:3098–3100
Vosko SH, Vilk L, Nusair M (1980) Can J Phys 58:1200–1211
Lee C, Yang W, Parr RG (1988) Phys Rev B 37:785–789
Njegic B, Gordon MS (2006) J Chem Phys 125:2241021–22410112
Materials Studio 4.1 (2004) Accelrys Inc, San Diego, CA
Kamlet MJ, Jacobs SJ (1968) J Chem Phys 48:23–35
Akhavan J (1998) Chemistry of explosives. The Royal Society of Chemistry, Cambridge
Zhang C, Shu Y, Huang Y, Zhao X, Dong H (2005) J Phys Chem B 109:8978–8982
Zhang C (2009) J Hazard Mater 161:21–28
Zhang C, Shu Y, Wang X, Zhao X, Tan B, Peng R (2005) J Phys Chem A 109:6592–6596
Zhang C, Shu Y, Huang Y, Wang X (2005) J Energ Mater 23:107–119
Fukui F, Yonezawa T, Shingu H (1952) J Chem Phys 20:722–725
Zhou Z, Parr RG (1990) J Am Chem Soc 112:5720–5724
Pearson RG (1989) J Org Chem 54:423–1430
Ravi P, Gore GM, Venkatesan V, Tewari SP, Sikder AK (2010) J Hazard Mater 183:859–865
Pinkerton AA, Zhuorva EA, Chen YS (2003) In: Politzer P, Murray JS (eds) Energetic Materials. Theoretical and Computational Chemistry Series. Elsevier, New York
Desiraju GR, Steiner T (1999) The weak hydrogen bond. Oxford University Press, New York
Bondi A (1964) J Phys Chem 68:441–451
Klapötke TM, Mayer P, Schulz A, Weigand JJ (2005) J Am Chem Soc 127:2032–2033
Cho SG, Goh EM, Kim JK (2001) Bull Korean Chem Soc 22:775–778
Politzer P, Martinez J, Murray JS, Concha MC, Toro-Labbe A (2009) Mol Phys 107:2095–2101
Kim CK, Cho SG, Kim CK, Park HY, Zhang H, Lee HW (2008) J Comput Chem 29:1818–8124
Belsky VK, Zorkii PM (1977) Acta Cryst A 13:1004–1006
Ravi P, Tewari SP (2012) Struct Chem. doi:10.1007/s11224-012-0028-9
Klapotke TM (2007) High energy density materials. Springer, Berlin
Politzer P, Murray JS (2011) Central Eur J Energ Mater 8:209–220
Depluech A, Cherville J (1978) Prop Explos Pyrotech 3:169–175
Depluech A, Cherville J (1979) Prop Explos Pyrotech 4:121–128
Xiao HM (1994) Molecular orbital theory of nitro compounds. Publishing House of Defense Industry, Peking
Kamlet MJ, Adolph HG (1979) Prop Explos Pyrotech 4:30–34
Mullay J (1987) Prop Explos Pyrotech 12:60–63
Politzer P, Murray JS (1995) Mol Phys 86:251–255
Murray JS, Concha MC, Politzer P (2009) Mol Phys 107:89–97
Rice BM, Hare JJ (2002) J Phys Chem A 106:1770–1783
Brinck T, Murray JS, Politzer P (1992) Mol Phys 76:609–617
Murray JS, Lane P, Politzer P (1995) Mol Phys 85:1–8
Murray JS, Lane P, Politzer P (1998) Mol Phys 93:187–194
Pospìŝil M, Vávra P, Concha MC, Murray JS, Politzer P (2010) J Mol Model 16:895–901
Zeman S (1999) J Energetic Mater 17:305–329
Zeman S (2006) J Hazard Mater 132:155–164
Manolopoulos DE, May JC, Down SE (1991) Chem Phys Lett 181:105–111
Hess BA Jr, Schaad LJ (1971) J Am Chem Soc 93:2413–2416
Haddon RC, Fukunaga T (1980) Tetrahedron Lett 21:191–1192
Schmalz TG, Seitz WA, Klein DJ, Hite GE (1988) J Am Chem Soc 110:1113–1127
Zhou Z, Parr RG, Garst JF (1988) Tetrahedron Lett 29:4843–4846
Zhou Z, Parr RG (1989) J Am Chem Soc 111:7371–7379
Liu X, Schmalz TG, Klein DJ (1992) Chem Phys Lett 188:550–554
Parr RG, Zhou Z (1993) Acc Chem Res 26:256–258
Aihara J, Oe S, Yoshida M, Ozawa E (1996) J Comput Chem 17:1387–1394
Aihara J (1999) J Phys Chem A 103:7487–7495
Acknowledgments
We are grateful to the referees for enlightening comments and useful suggestions. We thank Defense Research Development Organization, India for the financial assistance through Advanced Centre of Research in High Energy Materials.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ravi, P., Babu, B.K. & Tewari, S.P. Theoretical investigations on the structure, density, thermodynamic and performance properties of amino-, methyl-, nitroso- and nitrotriazolones. J Mol Model 19, 33–48 (2013). https://doi.org/10.1007/s00894-012-1515-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00894-012-1515-z