Abstract
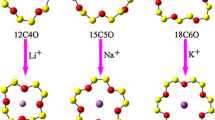
The crowned coumarin complexes are well known compounds for their ion recognition abilities. They undergo photophysical changes upon cation binding. On the basis of density functional theory calculations, we examined the sodium cation (Na+) binding energies of coumarin-crown ethers based on 15-Crown-5 (15 C5) and 18-Crown-6 (18 C6) as well as the optical absorptions of coumarin-crown ethers based on 12-Crown-4 (12 C4), 15 C5 and 18 C6. We explored why the attachment of crown ether ring to coumarin affects the Na+ binding energies of coumarin-crown ethers and also why the optical absorption of coumarin is modified by the crown ethers. Our study reveals that the Na+ ion binding energies of coumarin-crown ethers depend strongly on the size of the crown ether ring and also on the attachment position of the ether ring on coumarin. These factors affect the intramolecular charge transfer and overall stability of the complexes. The absorptions of the coumarin and ether ring parts of coumarin-crown ether are red shifted from those of isolated coumarin and crown ether, respectively. The red-shift of the coumarin ester group absorption is much stronger depending on the attachment position of the ether ring to coumarin. The absorption intensity of the coumarin part in coumarin-crown ethers is reduced for the benzene group absorption, but is enhanced for the ester group absorption.

In this study, the several Na+ - coumarin-crown ether coordination complexes based on 12-Crown-4, 15-Crown-5 and 18-Crown-6 were examined by the density functional theory method. The structural effects on the Na+ binding energies and optical properties were studied.



Similar content being viewed by others
References
Pedersen CJ (1967) Macrocyclic polyethers. J Am Chem Soc 89:2495–2500
Pedersen CJ (1967) Polyethers and their complexes with metal salts. J Am Chem Soc 89:7017–7036
Damewood JR, Anderson WP, Urban JJ (1988) A molecular mechanics study of netural molecule complexation with crown ethers. J Comput Chem 9:111–124
Erk Ç (2000) Cation recognition with fluorophore crown ethers. Ind Eng Chem Res 39:3582–3588
Blasius E, Cram DJ, Janzen KP, Müller WM, Sieger H, Trueblood KN, Vögtle F, Weber E (1981) Analytical applications of crown compounds and cryptands. Topics Curr Chem 98:1–189
Gokel G (1991) Crown Ethers and Cryptands. The Royal Society of Chemistry, Royal Society of Chemistry, Cambridge
Regueiro-Figuero M, Esteban-Gomez D, Platas-Iglesias C, de Blas A, Rodriguez-Blas T (2007) Metal ion comlpementarity: effect of rings size variation on the conformation and stability of lead(II) and cadmium(II) complexes with pendant-armed crowns. Eur J Inorg Chem 15:2198–2207
Inokuchi Y, Boyarkin OV, Kusaka R, Haino T, Ebata T, Rizzo TR (2011) UV and IR spectroscopic studies of cold alkali metal ıon_crown ether complexes in the gas phase. J Am Chem Soc 133:12256–12263
Gocmen A, Bulut M, Erk C (1993) A Synthesis and characterization of coumarin-crown ethers. Pure Appl Chem 65:447–450
Tiftikçi E, Erk C (2004) The synthesis of novel crown ethers, part X - 4-propyl- and 3-ethyl-4-methylchromenone-crown. J Heterocycl Chem 41:867–871
Ábalos T, Moragues M, Royo S,Jiménez D, Martínez-Máñez R,Soto J, Sancenón F, Gil S, Cano J (2012) Dyes that bear thiazolylazo groups as chromogenic chemosensors for metal cations. Eur J Inorg Chem 76–84
Anderson JD (2003) Alkali metal binding energies of dibenzo-18-crown-6: experimental and computational results. J Mass Spectrom 227:63–76
Islam MS, Pethrick RA, Pugh D, Wilson MJ (1997) Nuclear magnetic resonance and ab initio theoretical studies of 18-crown-6, benzo and dibenzo 18-crown-6 and their alkali-metal complexes. J Chem Soc Farad Transact 93(11):2097–2104
Kasapbasi E, Yurtsever M (2012) A DFT study on the structural and opticalp and cation selectivities of some metal-coumarin-crown ether complexes. Turk J Chem 36(1):147–158
Stephens PJ, Devlin FJ, Chabalowski CF, Frisch MJ (1994) Ab Initio calculation of vibrational absorption and circular dichroism spectra using density functional force fields. J Phys Chem 98:11623–11627
Stephens PJ, Devlin FJ, Ashvar CS, Bak KL, Taylor PR, Frisch MJ (1996) Comparison of local, nonlocal, and hybrid density functionals using vibrational absorption and circular dichroism spectroscopy. Chem Applıcatıons Densıty-Functıonal Theory Book Ser: Acs Symposıum Ser 629:105–113
Zhang Y, Wang X, LuoB XY (2012) DFT study of crown ether-bridged Z-stilbenes and their complexes with alkali metal cations. J Organomet Chem 699:31–38
Becke AD (1993) Density-functional thermochemistry. III. The role of exact exchange. J Chem Phys 98:5648–5652
Passos O, Fernandes PA (2011) Theoretical insights into the catalytic mechanism of beta-hexosaminidase. Theor Chem Acc 129:119–129
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Jr MontgomeryJA, Vreven T, Kudin KN, Burant JC, Millam JM, Iyengar SS, Tomasi J, Barone V, Mennucci B, Cossi M, Scalmani G, Rega N, Petersson GA, Nakatsuji H, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao H, Klene M, Li X, Knox JE, Hratchian HP, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Ayala PY, Morokuma K, Voth GA, Salvador P, Dannenberg JJ, Zakrzewski VG, Dapprich S, Daniels AD, Strain MC, Farkas O, Malick DK, Rabuck AD, Raghavachari K, Foresman JB, Ortiz JV, Cui Q, Baboul AG, Clifford S, Cioslowski J, Stefanov BB, Liu G, Liashenko A, Piskorz P, Komaromi I, Martin RL, Fox DJ, Keith T, Al-Laham MA, Peng CY, Nanayakkara A, Challacombe M, Gill PMW, Johnson B, Chen W, Wong MW, Gonzalez C, Pople JA (2004) Gaussian 03. Gaussian Inc, Wallingford
De Backer M, Hureau M, Depriester M, Deletoille A, Sargent AL, Forshee PB, Sibert JW (2008) On the oxidation of Wurster's reagent and the Wurster's crown analog of 15-crown-5 in the presence of alkali metal cations. J Electroanal Chem 612:97–104
Homem-de-Mello MBP, Tomasi J, da Silva ABF (2005) The effects of solvation in the theoretical spectra of cationic dyes. Theor Chem Acc 113:274–280
Tomasi J, Mennucci B, Cances E (1999) The IEF version of the PCM solvation method: an overview of a new method addressed to study molecular solutes at the QM ab initio level. J Mol Struct 464(1–3):211–226
Acknowledgments
We would like to acknowledge Prof. Dr. Mike H. Whangbo for careful reading of the manuscript
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOC 1.48 MB)
Rights and permissions
About this article
Cite this article
Kasapbasi, E., Yurtsever, M. Dependence of the optical absorption and Na+ binding energies of coumarin-crown ethers on the size and attachment position of ether ring: density functional investigation. J Mol Model 19, 173–178 (2013). https://doi.org/10.1007/s00894-012-1500-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00894-012-1500-6