Abstract
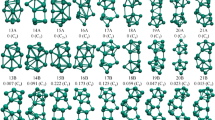
The present study reports the geometries, electronic structures, growth behavior, and stabilities of neutral and ionized copper-doped germanium clusters containing 1–20 Ge atoms within the framework of linear combination of atomic orbitals density functional theory (DFT) under the spin-polarized generalized gradient approximation. It was found that Cu-capped Ge n (or Cu-substituted Ge n+1) and Cu-encapsulated Ge n clusters mostly occur in the ground state at a particular cluster size (n). In order to explain the relative stabilities of the ground-state clusters, parameters such as the average binding energy per atom (BE), the embedding energy (EE), and the fragmentation energy (FE) of the clusters were calculated, and the resulting values are discussed. To explain the chemical stabilities of the clusters, parameters such as the energy gap between the highest occupied and the lowest unoccupied molecular orbitals (the HOMO–LUMO gap), the ionization energy (IP), the electron affinity (EA), the chemical potential (μ), the chemical hardness (η), and the polarizability were calculated, and the resulting values are also discussed. Natural atomic orbital (NAO) and natural bond orbital (NBO) analyses were also used to determine the electron-counting rule that should be applied to the most stable Ge10Cu cluster. Finally, the relevance of the calculated results to the design of Ge-based superatoms is discussed.


Contributions of the valance orbitals of the Ge and Cu atom(s) to the HOMO of the ground-state icosahedral Ge10Cu cluster obtained from NBO analysis. The numbers below the clusters represent the occupancies of the HOMO orbitals













Similar content being viewed by others
References
Brown WL, Freeman RR, Raghavachari K, Schluter M (1987) Science 235:860–865
Zhang X, Li G, Gao Z (2001) Rapid Comm Mass Spectrum 15:1573–1576
Khanna SN, Rao BK, Jena P (2002) Phys Rev Lett 89:016803–016806
Archibong EF, St-Amant A (1998) J Chem Phys 109:962–972
Benedict LX, Puzer A, Willimson AJ, Grossman JC, Galli G, Klepeis JE, Raty JY, Pankratov O (2003) Phys Rev B 68:85310–85318
Ho KM, Shvartzburg AA, Pan B, Lu ZY, Wang CZ, Wacker JG, Fye JL, Jarrold MF (1998) Nature 392:580–582
Shvartzburg AA, Jarrold MF (1999) Phys Rev A 60:1235–5
Jarrold MF, Bower JE (1992) J Chem Phys 96:9180–9190
Kumar V, Kawazoe Y (2001) Phys Rev Lett 87:045503–045504
Kumar V, Kawazoe Y (2002) Phys Rev Lett 88:235504–4
Bandyopadhyay D (2009) Nanotechnology 20:275202–275212
Rothlisberger U, Andreoni W, Parrinello M (1994) Phys Rev Lett 72:665–668
Kaxiras E, Jackson K (1993) Phys Rev Lett 71:727–730
Zdetsis AD (2007) Phys Rev B 76:075402–075405
Zhang D, Ma C, Lin C (2007) J Phys Chem C 111:17099–17103
Kumar V, Kawazoe Y (2007) Phys Rev B 75:155425–11
Beck SM (1987) J Chem Phys 87:4233–4234
Beck SM (1989) J Chem Phys 90:6306–6312
Hiura H, Miyazaki T, Kanayama T (2001) Phys Rev Lett 86:1733–1736
Ohara M, Miyajima K, Pramann A, Nakajima A, Kaya K (2002) J Phys Chem A 106:3702–3705
Bandyopadhyay D (2008) J Appl Phys 104:084308–7
Bandyopadhyay D (2009) Mol Simul 35:381–394
Bandyopadhyay D, Kumar M (2008) Chem Phys 353:170–176
Kumar M, Bhattacharrya N, Bandyopadhyay D (2012) J Mol Model 18:405–418
Bandyopadhyay D, Kaur P, Sen P (2010) J Phys Chem A 114:12986–12991
Bandyopadhyay D, Sen P (2010) J Phys Chem A 114:1835–1842
Gingerich KA, Schmude RW Jr, Baba MS, Meloni G (2000) J Chem Phys 112:7443–7448
Negishi Y, Kawamata H, Hayakawa F, Nakajima A, Kaya K (1998) Chem Phys Lett 294:370–376
Yoshida S, Fuke K (1999) J Chem Phys 111:3880–3890
Wang J, Chen X, Liu JH (2008) J Phys Chem A 112:8868–8876
Han JG (2000) Chem Phys Lett 324:143–148
Stroppa A, Kresse G, Continenza A (2011) Phys Rev B 83:085201–085205
Zhao WJ, Wang YX (2009) J Mol Struct (THEOCHEM) 901:18–23
Janssens E, Lievens P (2011) Adv Nat Sci Nanosci Nanotechnol 2:023001–023008
Negishi Y, Kawamata H, Hayase T, Gomei T, Kishi R, Hayakawa F, Nakajima A, Kaya K (1997) Chem Phys Lett 269:199–207
Huheey JE, Keiter EA, Keiter RL (2000) In: Inorganic chemistry: principles of structure and reactivity, 4th edn. HarperCollins, New York
Sen P, Mitas L (2003) Phys Rev B 68:155404–4
Reveles JU, Khanna SN (2005) Phys Rev B 72:165413–165418
Wigner E, Witmer EE (1928) Z Physik 51:859–886
Guo LJ, Zhao G, Gu Y, Liu X, Zeng Z (2008) Phys Rev B 77:195417–195418
Koyasu K, Akutsu M, Mitsui M, Nakajima A (2005) J Am Chem Soc 127:4998–4999
Perdew JP, Chevary JA, Vosko SH, Jackson KA, Pederson MR, Singh DJ, Fiolhais C (1992) Phys Rev B 46:6671–6678
Perdew JP, Chevary JA, Vosko SH, Jackson KA, Pederson MR, Singh DJ, Fiolhais C (1993) Phys Rev B 48:4978–4978
Perdew JP, Burke K, Wang Y (1996) Phys Rev B 54:16533–16537
Burke K, Perdew JP, Wang Y (1997) In: Dobson JF, Vignale G, Das MP (eds) Electronic density functional theory: recent progress and new directions. Plenum, New York, pp 28–111
Dunning TH Jr, Hay PJ (1976) In: Schaefer III HF (ed) Modern theoretical chemistry, vol 3. Plenum, New York, pp 1–28
Hay PJ, Wadt WR (1985) J Chem Phys 82:299–310
Fuentealba P, Preuss H, Stoll H, Szentpály LV (1982) Chem Phys Lett 89:418–422
Wang J, Han JG (2005) J Chem Phys 123:064306–064321
Han JG, Hagelberg F (2001) J Mol Struct (THEOCHEM) 549:165–180
Nagendran S, Sen SS, Roesky HW, Koley D, Grubmüller H, Pal A, Herbst-Irmer R (2008) Organometallics 27:5459–5463
Lombardi JR, Davis B (2002) Chem Rev 102:2431–2460
Morse MD (1993) Chemical bonding. In: The late transition metals: the nickel and copper group dimers, vol 1. JAI Inc., Greenwich
Wang YS, Chao SD (2011) J Phys Chem A 115:1472–1485
Khon W, Sham LJ (1965) Phys Rev 140:A1133–A1138
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Zakrzewski VG, Montgomery J A Jr, Stratmann RE, Burant JC, Dapprich S, Millam JM, Daniels AD, Kudin KN, Strain MC, Farkas O, Tomasi J, Barone V, Cossi M, Cammi R, Mennucci, B, Pomelli C, Adamo C, Clifford S, Ochterski J, Petersson GA, Ayala PY, Cui Q, Morokuma K, Malick DK, Rabuck AD, Raghavachari K, Foresman JB, Cioslowski J, Ortiz JV, Baboul AG, Stefanov BB, Liu B, Liashenko A, Piskorz P, Komaromi I, Gomperts R, Martin RL, Fox DJ, Keith T, Al-Laham MA, Peng CY, Nanayakkara A, Challacombe M, Gill PMW, Johnson B, Chen W, Wong MW, Andres JL, Gonzalez C, Head-Gordon M, Replogle ES, Pople JA (2004) Gaussian 03, revision E01. Gaussian Inc., Wallingford
de Heer WA (1993) Rev Mod Phys 65:611–676
Parr RG, Chattaraj PK (1991) J Am Chem Soc 113:1854–1855
Pearson RG (1987) J Chem Edu 64:561–567
Ayers PW, Parr RG (2008) J Chem Phys 128:184108–184116
Hati S, Datta D (1994) J Phys Chem 98:10451–10454
Ghanti TK, Ghosh SK (1994) J Phys Chem 98:9197–9201
Acknowledgments
Complete computations using Gaussian 03 were performed at the cluster computing facility, Harish-Chandra Research Institute, Allahabad, UP, India (http://cluster.hri.res.in).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bandyopadhyay, D. Architectures, electronic structures, and stabilities of Cu-doped Ge n clusters: density functional modeling. J Mol Model 18, 3887–3902 (2012). https://doi.org/10.1007/s00894-012-1374-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00894-012-1374-7