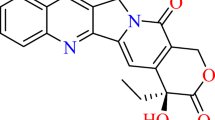
Abstract
Quinoline alkaloids are abundant in the Rutaceae, and many have exhibited cytotoxic activity. Because structurally related antitumor alkaloids such as camptothecin and fagaronine are known to function as intercalative topoisomerase poisons, it is hypothesized that cytotoxic Stauranthus alkaloids may also serve as intercalative topoisomerase inhibitors. To test this hypothesis theoretically, ten Stauranthus quinoline alkaloids were examined for potential intercalation into DNA using a molecular docking approach. Four of the alkaloids (stauranthine, skimmianine, 3′,6′-dihydroxy-3′,6′-dihydrostauranthine, and trans-3′,4′-dihydroxy-3′,4′-dihydrostauranthine) were able to intercalatively dock consistently into DNA. In order to probe the intermolecular interactions that may be responsible for intercalation of these quinoline alkaloids, density functional calculations have been carried out using both the B3LYP and M06 functionals. M06 calculations indicated favorable π–π interactions between either skimmianine or stauranthine and the guanine–cytosine base pair. Furthermore, the lowest-energy face-to-face orientation of stauranthine with guanine is consistent with favorable dipole–dipole orientations, favorable electrostatic interactions, and favorable frontier molecular orbital interactions. Likewise, the lowest-energy face-to-face orientation of stauranthine with the guanine–cytosine base pair reveals favorable electrostatic interactions as well as frontier molecular orbital interactions. Thus, not only can quinoline alkaloids dock intercalatively into DNA, but the docked orientations are also electronically favorable.

Lowest-energy face-to-face π–π interaction between stauranthine and guanine











Similar content being viewed by others
References
Wink M (2007) Alkaloids 64:1–47
Liu LF (1989) Annu Rev Biochem 58:351–375
Wilstermann AM, Osheroff N (2003) Curr Top Med Chem 3:321–338
Pommier Y, Pourquier P, Fan Y, Strumberg D (1998) Biochim Biophys Acta 1400:83–106
Burden DA, Osheroff N (1998) Biochim Biophys Acta 1400:139–154
Li QY, Zu YG, Shi RZ, Yao LP (2006) Curr Med Chem 13:2021–2039
Wang LK, Johnson RK, Hecht SM (1993) Chem Res Toxicol 6:813–818
Fox ME, Smith PJ (1990) Cancer Res 50:5813–5818
Stiborova M, Rupertova M, Schmeiser HH, Frei E (2006) Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub 150:13–23
Bonjean K, De Pauw-Gillet MC, Defresne MP, Colson P, Houssier C, Dassonneville L, Bailly C, Greimers R, Wright C, Quetin-Leclercq J, Tits M, Angenot L (1998) Biochemistry 37:5136–5146
Seigler DS (1977) Plant systematic and alkaloids. In: Manske RHF (ed) The alkaloids, vol XVI. Academic, New York, pp 1–82
Hu J, Zhang WD, Shen YH, Zhang C, Xu L, Liu RH, Wang B, Xu XK (2007) Biochem Syst Ecol 35:114–117
Boyd DR, Sharma ND, Loke PL, Malone JF, McRoberts WC, Hamilton JTG (2007) Org Biomol Chem 5:2983–2991
Svoboda GH, Poore GA, Simpson PJ, Boder GB (1966) J Pharm Sci 55:758–768
Wu TS, Wang ML, Jong TT, McPhail AT, McPhail DR, Lee KH (1989) J Nat Prod 52:1284–1289
Cui B, Chai H, Dong Y, Horgen FD, Hansen B, Madulid DA, Soejarto DD, Farnsworth NR, Cordell GA, Pezzuto JM, Kinghorn AD (1999) Phytochemistry 52:95–98
Chaturvedula VSP, Schilling JK, Miller JS, Andriantsiferana R, Rasamison VE, Kingston DGI (2003) J Nat Prod 66:532–534
Chen JJ, Fang HY, Duh CY, Chen IS (2005) Planta Med 71:470–475
Jansen O, Akhmedjanova V, Angenot L, Balansard G, Chariot A, Ollivier E, Tits M, Frédérich M (2006) J Ethnopharmacol 105:241–245
Prescott TAK, Sadler IH, Kiapranis R, Maciver SK (2007) J Ethnopharmacol 109:289–294
Kaczmarek L, Peczyńska-Czoch W, Osiadacz J, Mordarski M, Sokalski WA, Boratński J, Marcinkowska E, Glazman-Kuśnierczyk H, Radzikowski C (1999) Bioorg Med Chem 7:2457–2464
Osiadacz J, Majka J, Czarnecki K, Peczyńska-Czoch W, Zakrzewska-Czerwińska J, Kaczmarek Ł, Sokalski WA (2000) Bioorg Med Chem 8:937-943
Chen YL, Hung HM, Lu CM, Li KC, Tzeng CC (2004) Bioorg Med Chem 12:6539–6542
Carney JR, Scheuer PJ, Kelly-Borges M (1993) Tetrahedron 49(38):8483–8486
Molinski TF (1993) Chem Rev 93:1825–1838
McDonald LA, Eldredge GS, Barrows LR, Ireland CM (1994) J Med Chem 37:3819–3827
Dias N, Vezin H, Lansiaux A, Bailly C (2005) Top Curr Chem 253:89–108
Setzer WN, Setzer MC, Schmidt JM, Moriarity DM, Vogler B, Reeb S, Holmes AM, Haber WA (2000) Planta Med 66:493–494
Setzer WN, Vogler B, Bates RB, Schmidt JM, Dicus CW, Nakkiew P, Haber WA (2003) Phytochem Anal 14:54–59
Nunn CM, Van Meervelt L, Zhang SD, Moore MH, Kennard O (1991) J Mol Biol 222:167–177
Dautant A, Langlois d’Estaintot B, Gallois B, Brown T, Hunter WN (1995) Nucleic Acids Res 23:1710–1716
Berger I, Su L, Spitzner JR, Kang C, Burke TG, Rich A (1995) Nucleic Acids Res 23:4488–4494
Gao YG, Wang AH (1995) J Biomol Struct Dyn 13:103–117
Adams A, Guss JM, Collyer CA, Denny WA, Wakelin LPG (1999) Biochemistry 38:9221–9233
Robinson H, Gao YG, Yang XL, Sanishvili R, Joachimiak A, Wang AHJ (2001) Biochemistry 40:5587–5592
Smith CK, Davies GJ, Dodson EJ, Moore MH (1995) Biochemistry 34:415–425
Hu GG, Shui X, Leng F, Priebe W, Chaires JB, Williams LD (1997) Biochemistry 36:5940–5946
Lisgarten JN, Coll M, Portugal J, Wright CW, Aymami J (2002) Nature Struct Biol 9:57–60
Canals A, Purciolas M, Aymami J, Coll M (2005) Acta Crystallogr Sect D 61:1009–1012
Williams HEL, Colgrave ML, Searle MS (2002) Eur J Biochem 269:1726–1733
Robinson H, Priebe W, Chaires JB, Wang AH (1997) Biochemistry 36:8663–8670
Staker BL, Hjerrild K, Feese MD, Behnke CA, Burgin AB, Stewart LJ (2002) Proc Natl Acad Sci USA 99:15387–15392
Staker BL, Feese MD, Cushman M, Pommier Y, Zembower D, Stewart L, Burgin AB (2005) J Med Chem 48:2336–2345
Lauria A, Ippolito M, Almerico AM (2007) J Mol Model 13:393–400
Bhowmik S, Bagchi A, Ghosh R (2008) Int J Integr Biol 2:8–14
Byler KG (2001) Frontier molecular orbital interactions between intercalating quinoline alkaloids and DNA base pairs: an ab initio investigation. MS Thesis, University of Alabama in Huntsville
Nakatani K, Matsuno T, Adachi K, Hagihara S, Saito I (2001) J Am Chem Soc 123:5695–5702
Řeha D, Kabeláč M, Ryjáček F, Šponer J, Šponer JE, Elstner M, Suhai S, Hobza P (2002) J Am Chem Soc 124:3366–3376
Dračinský M, Castaño O (2004) Phys Chem Chem Phys 6:1799–1805
El-Gogary TM, Koehler G (2007) THEOCHEM 808:97–109
Kumar A, Elstner M, Suhai S (2003) Int J Quant Chem 95:44–59
Riahi S, Ganjali MR, Dinarvand R, Karamdoust S, Bagherzadeh K, Norouzi P (2008) Chem Biol Drug Des 71:474–482
Jena NR, Mishra PC (2007) J Mol Model 13:267–274
Hobza P, Šponer J (1999) Chem Rev 99:3247–3276
Hunter CA, Lawson KR, Perkins J, Urch CJ (2001) J Chem Soc Perkin Trans 2:651–669
Tsuzuki S, Honda K, Uchimaru T, Mikami M, Tanabe K (2002) J Am Chem Soc 124:104–112
Sinnokrot MO, Valeev EF, Sherrill CD (2002) J Am Chem Soc 124:10887–10893
Sato T, Tsuneda T, Hirao K (2005) J Chem Phys 123:104307
Podeszwa R, Bukowski R, Szalewicz K (2006) J Phys Chem A 110:10345–10354
DiStasio RA, von Helden G, Steele RP, Head-Gordon M (2007) Chem Phys Lett 437:277–283
Jha PC, Rinkevicius Z, Ågren H, Seal P, Chakrabarti S (2008) Phys Chem Chem Phys 10:2715–2712
Bludský O, Rubeš M, Soldán P, Nachtigall P (2008) J Chem Phys 128:114102
Pitoňák M, Riley KE, Neogrády P, Hobza P (2008) Chem Phys Chem 9:1636–1644
Dabkowska I, Gonzalez HV, Jurečka P, Hobza P (2005) J Phys Chem A 109:1131–1136
Cooper VR, Thonhauser T, Langreth DC (2008) J Chem Phys 128:204102
Šponer J, Riley KE, Hobza P (2008) Phys Chem Chem Phys 10:2595–2610
Jaffe RL, Smith GD (1996) J Chem Phys 105:2780–2788
Hobza P, Selzle HL, Schlag EW (1996) J Phys Chem 100:18790–18794
Tsuzuki S, Uchimaru T, Matsumura K, Mikami M, Tanabe K (2000) Chem Phys Lett 319:547–554
Tsuzuki S, Lüthi HP (2001) J Chem Phys 114:3949–3957
Milet A, Korona T, Moszynski R, Kochanski E (1999) J Chem Phys 111:7727–7735
Elstner M, Hobza P, Frauenheim T, Suhai S, Kaxiras E (2001) J Chem Phys 114:5149–5155
Cybulski SM, Bledson TM, Toczyłowski RR (2002) J Chem Phys 116:11039–11040
Mourik TV, Gdanitz RJ (2002) J Chem Phys 116:9620–9623
Cybulski SM, Seversen CE (2005) J Chem Phys 122:014117
Grimme S (2006) J Comput Chem 27:1787–1799
Jurečka P, Černý J, Hobza P, Salahub DR (2007) J Comput Chem 28:555–569
Wu Q, Yang W (2002) J Chem Phys 116:515–524
Antony J, Grimme S (2006) Phys Chem Chem Phys 8:5287–5293
Zhao Y, Truhlar DG (2004) J Phys Chem A 108:6908–6918
Zhao Y, Schultz NE, Truhlar DG (2006) J Chem Theory Comput 2:364–382
Zhao Y, Truhlar DG (2006) J Chem Phys 125:194101
Zhao Y, Truhlar DG (2005) Phys Chem Chem Phys 7:2701–2705
Dkhissi A, Blossey R (2007) Chem Phys Lett 439:35–39
Stepanian SG, Karachevtsev MV, Glamazda AYu, Karachevtsev VA, Adamowicz L (2008) Chem Phys Lett 459:153–158
Gu J, Wang J, Leszczynski J, Xie Y, Schaefer HF (2008) Chem Phys Lett 459:164–166
Wong BM (2009) J Comput Chem 30:51–56
Spartan ’08 for Windows (2006) Wavefunction, Irvine, CA
Halgren TA (1996) J Comp Chem 17:490–519
Thompson MA (2004) ArgusLab 4.0.1. Planaria Software LLC, Seattle, WA
Becke AD (1993) J Chem Phys 98:5648–5652
Lee C, Yang W, Parr RG (1988) Phys Rev B 37:785–789
Hehre WJ, Radom L, PvR S (1986) Ab initio molecular orbital theory. Wiley, New York
Chermette H (1999) J Comput Chem 20:129–154
Geerlings P, De Proft F, Langenaeker W (2003) Chem Rev 103:1793–1874
Sarkar U, Roy DR, Chattaraj PK, Parthasarathi R, Padmanabhan J, Subramanian V (2005) J Chem Sci 117:599–612
Chen JC, Qian L, Wu WJ, Chen LM, Zheng KC (2005) THEOCHEM 756:167–172
Chen JC, Shen Y, Liao S, Chen LM, Zheng KC (2007) Int J Quant Chem 107:1468–1478
Berman HM, Young PR (1981) Annu Rev Biophys Bioeng 10:87–114
Xiao S, Lin W, Wang C, Yang M (2001) Bioorg Med Chem Lett 11:437–441
El-Gogary TM, Koehler G (2009) THEOCHEM 895:57–64
Müller W, Crothers DM (1975) Eur J Biochem 54:267–277
Hunter CA, Lawson KR, Perkins J, Urch CJ (2001) J Chem Soc Perkin Trans 2:651–669
Boger DL, Invergo DJ, Coleman RS, Zarrinmayeh H, Kitos PA, Collins-Thompson S, Leong T, McLaughlin LW (1990) Chem Biol Interact 73:29–52
Haq I (2002) Arch Biochem Biophys 403:1–15
Baginski M, Fogolari F, Briggs JM (1997) J Mol Biol 274:253–267
Rehn C, Pindur U (1996) Monats Chem 127:645–658
Nakatani K, Matsuno T, Adachi K, Hagihara S, Saito I (2001) J Am Chem Soc 123:5695–5702
Mei WJ, Liu J, Zheng KC, Lin LJ, Chao H, Li AX, Yun FC, Ji LN (2003) Dalton Trans 2003:1352–1359
Nowak K, Wysocki S (2004) THEOCHEM 682:191–199
Fukui K, Yonezawa T, Shingu H (1952) J Chem Phys 20:722–725
Pullman B (1991) Anticancer Drug Design 6:95–105
Trotta E, D’Ambrosio E, Ravagnan G, Paci M (1995) Nucleic Acids Res 23:1333–1340
Rehn C, Pundur U (1996) Monats Chem 127:631–644
Lisgarten JN, Coll M, Portugal J, Wright CW, Aymami J (2002) Nature Struct Biol 9:57–60
Ghose AK, Pritchett A, Crippen GM (1988) J Comput Chem 9:80–90
Cramer CJ, Truhlar DG (1992) J Comput Chem 13:1089–1097
Chambers CC, Hawkins GD, Cramer CJ, Truhlar DG (1996) J Phys Chem 100:16385–16398
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Byler, K.G., Wang, C. & Setzer, W.N. Quinoline alkaloids as intercalative topoisomerase inhibitors. J Mol Model 15, 1417–1426 (2009). https://doi.org/10.1007/s00894-009-0501-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00894-009-0501-6