Abstract
We have carried out B3PW91 and MP2-FC computational studies of dimethyl sulfoxide, (CH3)2SO, and dimethyl sulfone, (CH3)2SO2. The objective was to establish quantitatively the basis for their high polarities and boiling points, and their strong solvent powers for a variety of solutes. Natural bond order analyses show that the sulfur–oxygen linkages are not double bonds, as widely believed, but rather are coordinate covalent single S+→O− bonds. The calculated electrostatic potentials on the molecular surfaces reveal several strongly positive and negative sites (the former including σ-holes on the sulfurs) through which a variety of simultaneous intermolecular electrostatic interactions can occur. A series of examples is given. In terms of these features the striking properties of dimethyl sulfoxide and dimethyl sulfone, their large dipole moments and dielectric constants, their high boiling points and why they are such good solvents, can readily be understood.

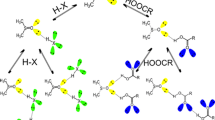
Dimers of dimethyl sulfoxide (DMSO; left) and dimethyl sulfone (DMSO2; right) showing O S—O -hole bonding and C H—O hydrogen bonding. Sulfur atoms are yellow, oxygens are red, carbons are gray and hydrogens are white









Similar content being viewed by others
References
Lide DR (ed) (2006) Handbook of Chemistry and Physics, 87th edn. CRC, Boca Raton, FL
Gaylord Chemical Corp., Research and Technology Center, Bogalusa, LA
Johnson III RD (ed) (2005) NIST Computational Chemistry Comparison and Benchmark Database, NIST Standard Reference Database No. 101, Release 12, http://srdata.nist.gov/cccbdb
Windholz M (ed) (1983) The Merck Index, 10th edn, Merck, Rahway, NJ
Stewart RF (1979) Chem Phys Lett 65:335–342
Politzer P, Truhlar DG (eds) (1981) Chemical Applications of Atomic and Molecular Electrostatic Potentials. Plenum, New York
Bader RFW, Carroll MT, Cheeseman JR, Chang C (1987) J Am Chem Soc 109:7968–7979
Hagelin H, Murray JS, Brinck T, Berthelot M, Politzer P (1995) Can J Chem 73:483–488
Murray JS, Politzer P (1998) J Mol Struct (Theochem) 425:107–114
Politzer P, Murray JS (1999) Trends Chem Phys 7:157–165
Reed AE, Curtiss LA, Weinhold F (1988) Chem Rev 88:899–926
Grimme S (2006) J Comput Chem 27:1787–1799
Arndt F, Eistert B (1941) Ber Dtsch Chem Ges 74:451–459
Phillips GM, Hunter JS, Sutton LE (1945) J Chem Soc 146–162
Moffitt W (1950) Proc R Soc A 200:409–428
Cruickshank DW (1961) J Chem Soc 5486–5504
Clark T, Hennemann M, Murray JS, Politzer P (2007) J Mol Model 13:291–296
Politzer P, Lane P, Concha MC, Ma Y, Murray JS (2007) J Mol Model 13:305–311
Murray JS, Lane P, Politzer P (2007) Int J Quantum Chem 107:2286–2292
Murray JS, Lane P, Clark T, Politzer P (2007) J Mol Model 13:1033–1038
Politzer P, Murray JS, Concha MC (2007) J Mol Model 13:643–650
Bernard-Houplain MC, Sandorfy C (1973) Can J Chem 51(1075):3640–3646
Di Paolo T, Sandorfy C (1974) Can J Chem 52:3612–3622
Rosenfield Jr RE, Parthasarathy R, Dunitz JD (1977) J Am Chem Soc 99:4860–4862
Murray-Rust P, Motherwell WDS (1979) J Am Chem Soc 101:4374–4376
Guru Row TN, Parthasarathy R (1981) J Am Chem Soc 103:477–479
Ramasubbu N, Parthasarathy R, Murray-Rust P (1986) J Am Chem Soc 108:4308–4314
Iwaoka M, Komatsu H, Katsuda T, Tomoda S (2002) J Am Chem Soc 124:1902–1909 and papers cited
Lommerse JPM, Stone AJ, Taylor R, Allen FH (1996) J Am Chem Soc 118:3108–3116
Valerio G, Raos G, Meille SV, Metrangolo P, Resnati G (2000) J Phys Chem A 104:1617–1620
Romaniello P, Lelj F (2002) J Phys Chem A 106:9114–9119
Cozzolino AF, Vargas-Baca I, Mansour S, Mahmoudkhani AH (2005) J Am Chem Soc 127:3184–3190
Bleiholder C, Werz DB, Köppel H, Gleiter R (2006) J Am Chem Soc 128:2666–2674
Corradi E, Meille SV, Messina MT, Metrangolo P, Resnati G (2000) Angew Chem Int Ed 39:1782–1786
Politzer P, Murray JS, Lane P (2007) Int J Quantum Chem 107:3046–3052
Bondi A (1964) J Phys Chem 68:441–451
Onthong U, Megyes T, Bakó I, Radnai T, Grósz T, Hermansson K, Probst M (2004) Phys Chem Chem Phys 6:2136–2144
Acknowledgment
This work was supported in part by the Deutsche Forschungsgemeinschaft as part of SFB583 Redox-active Metal Complexes: Control of Reactivity via Molecular Architecture.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Clark, T., Murray, J.S., Lane, P. et al. Why are dimethyl sulfoxide and dimethyl sulfone such good solvents?. J Mol Model 14, 689–697 (2008). https://doi.org/10.1007/s00894-008-0279-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00894-008-0279-y