Abstract
The inner mechanism and dynamic stereochemistry of electrophilic addition of bromine to bisbenzotetracyclo[6.2.2.23,6.02,7]tetradeca-4,9,11,13-tetraene(BBTT) molecule have been investigated by the methods of quantum chemistry. The structure of the BBTT molecule has been studied by ab initio and DFT/B3LYP methods using the 6-31G(d) and 6-311G(d) basis sets. The double bonds of BBTT molecule are endo-pyramidalized. The structure and stability of the cationic intermediates and products of the addition reaction have been investigated by HF/6-311G(d), HF/6-311G(d,p), B3LYP/6-311G(d) and B3LYP/6-311++G(2d,p)//B3LYP/6-311G(d) methods. The bridged bromonium cation isomerized into the more stable nonclassical delocalized N- and U-type cations and the difference between the stability of these cations is small. For the determination of the direction of addition reaction and the stereochemistry of the products, the stability of nonclassical delocalized N- and U-type ions and the structure of their cationic centres play a vital role. Since the cationic centre of the N-type ion is in interaction with the benzene ring from the exo face, the nucleofilic attackof the bromide anion to this centre occurs from the endo face and the exo,endo-isomer of the N-type product is obtained. The attack of bromide anion, towards the cationic centre of U-type ion from the endo face is sterically hindered by the hydrogen atom therefore the attack occurs from the exo face, which interacts with the benzene ring and the more stable exo,exo-isomer of U-type product is formed. Although, the U-type cation was 2.232 kcal mol−1 more stable than the N-type cation, the U-type product was 0.587 kcal mol−1 less stable than the N-type product.

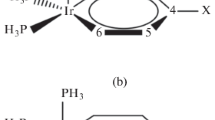
The energy diagram of BBTT-Br2 system (kcal mol−1) [B3LYP/6-311++G(2d,p)//B3LYP/6-311G(d)]




Similar content being viewed by others
References
Goldstein MJ, Hoffmann R (1971) J Am Chem Soc 93:6193–6204
Gleiter R, Schafer W (1990) Acc Chem Res 23:369–375
Hoffmann R, Imamura A, Hehre WJ (1968) J Am Chem Soc 90:1499–1509
Hoffmann R (1971) Acc Chem Res 4:1–9
Grimme W, Wortmann J, Frowein D, Lex J, Chen G, Gleiter R (1998) J Chem Soc Perkin Trans 2:1893–1900
Osawa E, Aigami K, Inamoto Y (1978) Tetrahedron 34:509–515
Lin CT, Wang NJ, Yeh YL, Chou TC (1995) Tetrahedron 51:2907–2928
Lin CT, Wang NJ, Tseng HZ, Chou TC (1997) J Org Chem 62:4857–4861
Lin CT, Hsu HC, Chou TC (1999) J Org Chem 64:7260–7264
Soloway SB, Damiana AM, Sim JW, Bluestone H, Lidov RE (1960) J Am Chem Soc 82:5377–5385
Franz HJ, Hobold W, Hohn R, Muller-Hagen G, Muller R, Pritzkow R, Schmidt H (1970) J Prakt Chem 320:622–634
Haufe G, Kleinpeter E, Muhlstadt M, Graefe J (1978) Monatshefte f. Chem 109:575–585
Matturro MG, Adams RD, Wiberg KB (1981) Chem Commun 17:878–879
Uemura S, Fukuzawa S, Toshimitsu A, Masaya O (1983) J Org Chem 48:270–273
Wiberg KB, Adams RD, Okarma PJ, Matturro MG, Segmıller B (1984) J Am Chem Soc 6:2200–2206
Kimura M, Morossawa S (1985) J Org Chem 50:1532–1534
Shea KJ, Greeley AC, Nguyen S, Beauchamp PD, Aue DH, Witzeman JS (1986) J Am C Chem Soc 108:5901–5908
Haufe G, Alvernhe G, Laurent A (1986) Tetrahedron Lett 4449–4452
Murty BARC, Pinkos R, Spurr PR, Fessner WD, Lutz G, Fritz H, Hunkler D, ri Prinzbach H (1992) Chem Ber 125:1719–1739
Pinkos R, Melder JP, Weber K, Hunkler D, Prinzbach H (1993) J Am Chem Soc 115:7173–7191
Herges R, Neumann H (1995) Liebigs Ann 1283–1289
Robinson RE, Myers DY (1999) Tetrahedron Lett 1099–1100
Günbas DD, Algı F, Hökelek T, Watson WH, Balcı M (2005) Tetrahedron 61:11177–11183
Inagaki S, Fujimoto H, Fukui K (1976) J Am Chem Soc 98:4054–4061
Belluci G, Chiappe C, Bianchini R, Lenoir D, Herges RJ (1995) J Am Chem Soc 117:12001–12002
Herges R (1995) Angew Chem Int Ed Engl 34:51–53
Ruiz E, Dennis R, Salahub R, Vela A (1996) J Phys Chem 100:12265–12276
Brown RS (1997) Acc Chem Res 30(3):131–137
Bianchini R, Chiappe C, Lenoir D, Lemmen P, Herges R, Grunenberg J (1997) Angeew Chem Int Ed Eng 36:1284–1287
Smith WB (1998) J Org Chem 63:2661–2664
Bianchini R, Chiappe C, Moro LG, Lenoir D, Lemmen P, Goldberg N (1999) Chem Eur J 5:1570–1580
Chiappe C, Rubertis AD, Lemmen P, Lenoir D (2000) J Org Chem 65:1273–1279
Chiappe C, Rubertis AD, Detert H, Lenoir D, Wannere C, Schleyer RP (2002) Chem Eur J 8:967–978
Rathere R, Lindeman SV, Zhu CJ, Mori T, Schleyer RP, Kochi JK (2002) J Org Chem 67:5106–5116
Lenoir D, Chiappe C (2003) Chem Eur J 9:1037–1044
Chiappe C, Detert H, Lenoir D, Pomelli CS, Ruasse MF (2003) J Am Chem Soc 125:2864–2865
Herges R, Papafflippopoulos A, Hess K, Chiappe C, Lenoir D, Detert H (2005) Angew Chem Int Ed 44:1412–1416
Chiappe C, Pomelli CS, Lenoir D, Wattenbach C (2006) J Mol Model 12:631–639
Abbasoglu R (2004) J Mol Struct (Theochem) 686:1–5 and references therein
Abbasoglu R, Yilmaz S, Gök Y (2005) Indian J Chem 44A:221–226
Abbasoglu R, Yilmaz S (2006) J Mol Model 12:290–296
Abbasoglu R (2006) J Mol Model 12:991–995
Abbasoglu R (2007) J Mol Model 13:425–430
Lee C, Yang W, Parr RG (1988) Phys Rev B 37:785–789
Becke AD (1993) J Chem Phys 98:5648–5652
Hehre WJ, Ditchfield R, Pople JA (1972) J Chem Phys 56:2257–2261
Krishnan R, Binkley JS, Seeger R, Pople JA (1980) J Chem Phys 72:650–654
Borden WT (1989) Chem Rev 89:1095–1109
Ermer O, Bell P, Mason SA (1989) Angew Chem Int Ed Engl 28:1239–1241
Osawa E, Aigami K, Inamoto Y (1977) J Org Chem 42:2622–2626
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Abbasoglu, R. Ab initio and DFT study of the inner mechanism and dynamic stereochemistry of electrophilic addition reaction of bromine to bisbenzotetracyclo[6.2.2.23,6.02,7]tetradeca-4,9,11,13-tetraene. J Mol Model 13, 1215–1220 (2007). https://doi.org/10.1007/s00894-007-0236-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00894-007-0236-1