Abstract
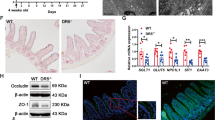
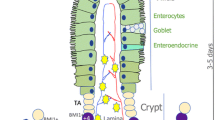
Homeostasis of the continuously self-renewing intestinal tract involves cell proliferation, migration, differentiation along the crypt-villus-axis and shedding of cells into the gut lumen. CD95-ligand (FAS-ligand, CD95L) is a cytokine that is known for its capacity to induce apoptosis by binding its cognate receptor, CD95 (Fas). More recently, it was discovered that CD95L can also induce other cellular responses, such as proliferation, differentiation and cell migration. CD95L is highly expressed in Paneth cells of the small intestine which are in close contact with intestinal stem cells. This suggests a potential role for CD95L in controlling stem cell function and, possibly, intestinal homeostasis. We analyzed the intestines of mice deficient for functional CD95L (gld) for potential alterations in the diversity of stem-cell-lineages and parameters of intestinal homeostasis. Stem cell diversity was assessed by analyzing methylation patterns of the non-transcribed mMYOD gene. Proliferation was analyzed by BrdU labeling and differentiation was assessed by immunohistochemistry. Of all parameters analyzed, only epithelial cell proliferation was significantly reduced in the small intestines of gld-mice, but not in their colons which lack CD95L expression. We conclude that CD95L has a proliferation-stimulating role during normal turnover of the small intestine, but has a marginal effect on overall intestinal homeostasis.




Similar content being viewed by others
Abbreviations
- CD95L:
-
CD95 ligand
- BrdU:
-
Bromodeoxyuridine
References
van der Flier LG, Clevers H (2009) Stem cells, self-renewal, and differentiation in the intestinal epithelium. Annu Rev Physiol 71:241–260
Chen L, Park SM, Turner JR, Peter ME (2010) Cell death in the colonic epithelium during inflammatory bowel diseases: CD95/Fas and beyond. Inflamm Bowel Dis 16:1071–1076
Steller EJ, Borel Rinkes IH, Kranenburg O (2011) How CD95 stimulates invasion. Cell Cycle 10:3857–3862
Corsini NS, Sancho-Martinez I, Laudenklos S, Glagow D, Kumar S, Letellier E et al (2009) The death receptor CD95 activates adult neural stem cells for working memory formation and brain repair. Cell Stem Cell 5:178–190
Barca O, Seoane M, Senaris RM, Arce VM (2013) Fas/CD95 ligation induces proliferation of primary fetal astrocytes through a mechanism involving caspase 8-mediated ERK activation. Cell Physiol Biochem 32:111–120
Li H, Cai X, Fan X, Moquin B, Stoicov C, Houghton J (2008) Fas Ag-FasL coupling leads to ERK1/2-mediated proliferation of gastric mucosal cells. Am J Physiol Gastrointest Liver Physiol 294:G263–G275
Leithauser F, Dhein J, Mechtersheimer G, Koretz K, Bruderlein S, Henne C et al (1993) Constitutive and induced expression of APO-1, a new member of the nerve growth factor/tumor necrosis factor receptor superfamily, in normal and neoplastic cells. Lab Invest 69:415–429
Moller P, Walczak H, Reidl S, Strater J, Krammer PH (1996) Paneth cells express high levels of CD95 ligand transcripts: a unique property among gastrointestinal epithelia. Am J Pathol 149:9–13
Strater J, Moller P (2003) CD95 (Fas/APO-1)/CD95L in the gastrointestinal tract: fictions and facts. Virchows Arch 442:218–225
Cohen PL, Eisenberg RA (1991) Lpr and gld: single gene models of systemic autoimmunity and lymphoproliferative disease. Annu Rev Immunol 9:243–269
Sato T, van Es JH, Snippert HJ, Stange DE, Vries RG, van den Born M et al (2011) Paneth cells constitute the niche for Lgr5 stem cells in intestinal crypts. Nature 469:415–418
Zuliani C, Kleber S, Klussmann S, Wenger T, Kenzelmann M, Schreglmann N et al (2006) Control of neuronal branching by the death receptor CD95 (Fas/Apo-1). Cell Death Differ 13:31–40
Hirano K, Sasaki N, Ichimiya T, Miura T, Van Kuppevelt TH, Nishihara S (2012) 3-O-sulfated heparan sulfate recognized by the antibody HS4C3 contribute to the differentiation of mouse embryonic stem cells via Fas signaling. PLoS ONE 7:e43440
Takahashi T, Tanaka M, Brannan CI, Jenkins NA, Copeland NG, Suda T et al (1994) Generalized lymphoproliferative disease in mice, caused by a point mutation in the Fas ligand. Cell 76:969–976
Mashimo H, Wu DC, Podolsky DK, Fishman MC (1996) Impaired defense of intestinal mucosa in mice lacking intestinal trefoil factor. Science 274:262–265
Arasaradnam RP, Quraishi MN, Commane D, Mathers JC, Bradburn M (2012) MYOD-1 in normal colonic mucosa–role as a putative biomarker? BMC Res Notes 5:240
Chu MW, Siegmund KD, Eckstam CL, Kim JY, Yang AS, Kanel GC et al (2007) Lack of increases in methylation at three CpG-rich genomic loci in non-mitotic adult tissues during aging. BMC Med Genet 8:50
Yatabe Y, Tavare S, Shibata D (2001) Investigating stem cells in human colon by using methylation patterns. Proc Natl Acad Sci USA 98:10839–10844
Kim KM, Calabrese P, Tavare S, Shibata D (2004) Enhanced stem cell survival in familial adenomatous polyposis. Am J Pathol 164:1369–1377
Langeveld D, Jansen M, de Boer DV, van Sprundel M, Brosens LA, Morsink FH et al (2012) Aberrant intestinal stem cell lineage dynamics in Peutz-Jeghers syndrome and familial adenomatous polyposis consistent with protracted clonal evolution in the crypt. Gut 61:839–846
Nicolas P, Kim KM, Shibata D, Tavare S (2007) The stem cell population of the human colon crypt: analysis via methylation patterns. PLoS Comput Biol 3:e28
Zak S, Treven J, Nash N, Gutierrez LS (2008) Lack of thrombospondin-1 increases angiogenesis in a model of chronic inflammatory bowel disease. Int J Colorectal Dis 23:297–304
Zamai L, Ahmad M, Bennett IM, Azzoni L, Alnemri ES, Perussia B (1998) Natural killer (NK) cell-mediated cytotoxicity: differential use of TRAIL and Fas ligand by immature and mature primary human NK cells. J Exp Med 188:2375–2380
Lee RK, Spielman J, Zhao DY, Olsen KJ, Podack ER (1996) Perforin, Fas ligand, and tumor necrosis factor are the major cytotoxic molecules used by lymphokine-activated killer cells. J Immunol 157:1919–1925
Strater J, Moller P (2000) Expression and function of death receptors and their natural ligands in the intestine. Ann N Y Acad Sci 915:162–170
Bjerknes M, Cheng H (1999) Clonal analysis of mouse intestinal epithelial progenitors. Gastroenterology 116:7–14
Snippert HJ, van der Flier LG, Sato T, van Es JH, van den Born M, Kroon-Veenboer C et al (2010) Intestinal crypt homeostasis results from neutral competition between symmetrically dividing Lgr5 stem cells. Cell 143:134–144
Hoogwater FJ, Steller EJ, Westendorp BF, Borel Rinkes IH, Kranenburg O (2012) CD95 signaling in colorectal cancer. Biochim Biophys Acta 1826:189–198
Park SM, Chen L, Zhang M, Ashton-Rickardt P, Turner JR, Peter ME (2010) CD95 is cytoprotective for intestinal epithelial cells in colitis. Inflam Bowel Dis 16:1063–1070
Desbarats J, Newell MK (2000) Fas engagement accelerates liver regeneration after partial hepatectomy. Nat Med 6:920–923
Reinehr R, Sommerfeld A, Haussinger D (2008) CD95 ligand is a proliferative and antiapoptotic signal in quiescent hepatic stellate cells. Gastroenterology 134:1494–1506
Marshman E, Ottewell PD, Potten CS, Watson AJ (2001) Caspase activation during spontaneous and radiation-induced apoptosis in the murine intestine. J Pathol 195:285–292
Vyas D, Robertson CM, Stromberg PE, Martin JR, Dunne WM, Houchen CW et al (2007) Epithelial apoptosis in mechanistically distinct methods of injury in the murine small intestine. Histol Histopathol 22:623–630
Lavrik IN, Golks A, Riess D, Bentele M, Eils R, Krammer PH (2007) Analysis of CD95 threshold signaling: triggering of CD95 (FAS/APO-1) at low concentrations primarily results in survival signaling. J Biol Chem 282:13664–13671
Legembre P, Barnhart BC, Zheng L, Vijayan S, Straus SE, Puck J et al (2004) Induction of apoptosis and activation of NF-κB by CD95 require different signalling thresholds. EMBO Rep 5:1084–1089
Acknowledgments
The authors thank all members of the Surgical Oncology research group for the input and constructive feedback during work discussions. The authors also thank the employees of the Pathology department and in particular P. van de Groep, D. Castigliego, P. Homoet and M. Huynh for their technical assistance and Marco Koudijs and Nicolle Besselink for the excellent technical assistance and knowledge of the Ion Torrent Platform. This study was supported by the PON foundation (KT; EJAS), “Vrienden UMC Utrecht” foundation (KT) and the Dutch Cancer Society (EJAS).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Trumpi, K., Steller, E.J.A., de Leng, W.W. et al. Mice lacking functional CD95-ligand display reduced proliferation of the intestinal epithelium without gross homeostatic alterations. Med Mol Morphol 49, 110–118 (2016). https://doi.org/10.1007/s00795-015-0129-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00795-015-0129-9