Abstract
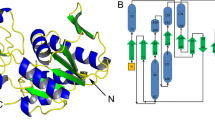
The enzyme 5-phosphoribosyl-1-α-diphosphate (PRPP) synthase (EC 2.7.6.1) catalyses the Mg2+-dependent transfer of a diphosphoryl group from ATP to the C1 hydroxyl group of ribose 5-phosphate resulting in the production of PRPP and AMP. A nucleotide sequence specifying Sulfolobus solfataricus PRPP synthase was synthesised in vitro with optimised codon usage for expression in Escherichia coli. Following expression of the gene in E. coli PRPP synthase was purified by heat treatment and ammonium sulphate precipitation and the structure of S. solfataricus PRPP synthase was determined at 2.8 Å resolution. A bent dimer oligomerisation was revealed, which seems to be an abundant feature among PRPP synthases for defining the adenine specificity of the substrate ATP. Molecular replacement was used to determine the S. solfataricus PRPP synthase structure with a monomer subunit of Methanocaldococcus jannaschii PRPP synthase as a search model. The two amino acid sequences share 35 % identity. The resulting asymmetric unit consists of three separated dimers. The protein was co-crystallised in the presence of AMP and ribose 5-phosphate, but in the electron density map of the active site only AMP and a sulphate ion were observed. Sulphate ion, reminiscent of the ammonium sulphate precipitation step of the purification, seems to bind tightly and, therefore, presumably occupies and blocks the ribose 5-phosphate binding site. The activity of S. solfataricus PRPP synthase is independent of phosphate ion.



Similar content being viewed by others
Abbreviations
- PRPP:
-
5-Phospho-d-ribosyl 1-α-diphosphate
References
Afonine PV, Grosse-Kunstleve RW, Adams PD (2005) The phenix refinement framework. CCP4 Newslett 42:8
Alderwick LJ, Lloyd GS, Lloyd AJ, Lovering AL, Eggeling L, Besra GS (2011) Biochemical characterization of the Mycobacterium tuberculosis phosphoribosyl-1-pyrophosphate synthetase. Glycobiology 21:410–425. doi:10.1093/glycob/cwq173
Arnvig K, Hove-Jensen B, Switzer RL (1990) Purification and properties of phosphoribosyl-diphosphate synthetase from Bacillus subtilis. Eur J Biochem 192:195–200
Breda A, Martinelli LK, Bizarro CV, Rosado LA, Borges CB, Santos DS, Basso LA (2012) Wild-type phosphoribosylpyrophosphate synthase (PRS) from Mycobacterium tuberculosis: a bacterial class II PRS? PLoS One 7:e39245. doi:10.1371/journal.pone.0039245
Cherney MM, Cherney LT, Garen CR, James MN (2011) The structures of Thermoplasma volcanium phosphoribosyl pyrophosphate synthetase bound to ribose-5-phosphate and ATP analogs. J Mol Biol 413:844–856. doi:10.1016/j.jmb.2011.09.007
Collaborative Computational Projekt Number 4 (1994) The CCP4 suite: programs for protein crystallography. Acta Crystallogr D Biol Crystallogr 50:760–763
Corpet F (1988) Multiple sequence alignment with hierarchical clustering. Nucleic Acids Res 16:10881–10890
Emsley P, Cowtan K (2004) Coot: model-building tools for molecular graphics. Acta Crystallogr D Biol Crystallogr 60:2126–2132. doi:10.1107/S0907444904019158
Eriksen TA, Kadziola A, Bentsen AK, Harlow KW, Larsen S (2000) Structural basis for the function of Bacillus subtilis phosphoribosyl-pyrophosphate synthetase. Nat Struct Biol 7:303–308. doi:10.1038/74069
Eriksen TA, Kadziola A, Larsen S (2002) Binding of cations in Bacillus subtilis phosphoribosyldiphosphate synthetase and their role in catalysis. Protein Sci 11:271–279. doi:10.1110/ps.28502
Hove-Jensen B (1988) Mutation in the phosphoribosylpyrophosphate synthetase gene (prs) that results in simultaneous requirements for purine and pyrimidine nucleosides, nicotinamide nucleotide, histidine, and tryptophan in Escherichia coli. J Bacteriol 170:1148–1152
Hove-Jensen B (1989) Phosphoribosylpyrophosphate (PRPP)-less mutants of Escherichia coli. Mol Microbiol 3:1487–1492
Hove-Jensen B, Maigaard M (1993) Escherichia coli rpiA gene encoding ribose phosphate isomerase A. J Bacteriol 175:5628–5635
Hove-Jensen B, Harlow KW, King CJ, Switzer RL (1986) Phosphoribosylpyrophosphate synthetase of Escherichia coli. Properties of the purified enzyme and primary structure of the prs gene. J Biol Chem 261:6765–6771
Huang H, Scherman MS, D’Haeze W, Vereecke D, Holsters M, Crick DC, McNeil MR (2005) Identification and active expression of the Mycobacterium tuberculosis gene encoding 5-phospho-α-d-ribose-1-diphosphate: decaprenyl-phosphate 5-phosphoribosyltransferase, the first enzyme committed to decaprenylphosphoryl-d-arabinose synthesis. J Biol Chem 280:24539–24543. doi:10.1074/jbc.M504068200
Jensen KF, Houlberg U, Nygaard P (1979) Thin-layer chromatographic methods to isolate 32P-labeled 5-phosphoribosyl-α-1-pyrophosphate (PRPP): determination of cellular PRPP pools and assay of PRPP synthetase activity. Anal Biochem 98:254–263
Jensen KF, Dandanell G, Hove-Jensen B, Willemoës M (2008) Chapter 3.6.2, Nucleotides, nucleosides, and nucleobases. ASM Press. Accessed 18 August
Kadziola A, Jepsen CH, Johansson E, McGuire J, Larsen S, Hove-Jensen B (2005) Novel class III phosphoribosyl diphosphate synthase: structure and properties of the tetrameric, phosphate-activated, non-allosterically inhibited enzyme from Methanocaldococcus jannaschii. J Mol Biol 354:815–828
Kornberg A, Lieberman I, Simms ES (1955) Enzymatic synthesis and properties of 5-phosphoribosylpyrophosphate. J Biol Chem 215:389–402
Krath BN, Hove-Jensen B (1996) Bacillus caldolyticus prs gene encoding phosphoribosyl-diphosphate synthase. Gene 176:73–79
Krath BN, Hove-Jensen B (1999) Organellar and cytosolic localization of four phosphoribosyl diphosphate synthase isozymes in spinach. Plant Physiol 119:497–506
Krath BN, Hove-Jensen B (2001a) Class II recombinant phosphoribosyl diphosphate synthase from spinach. Phosphate independence and diphosphoryl donor specificity. J Biol Chem 276:17851–17856
Krath BN, Hove-Jensen B (2001b) Implications of secondary structure prediction and amino acid sequence comparison of class I and class II phosphoribosyl diphosphate synthases on catalysis, regulation, and quaternary structure. Protein Sci 10:2317–2324. doi:10.1110/ps.11801
Krath BN, Eriksen TA, Poulsen TS, Hove-Jensen B (1999) Cloning and sequencing of cDNAs specifying a novel class of phosphoribosyl diphosphate synthase in Arabidopsis thaliana. Biochim Biophys Acta 1430:403–408
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685
Li S, Lu Y, Peng B, Ding J (2007) Crystal structure of human phosphoribosylpyrophosphate synthetase 1 reveals a novel allosteric site. Biochem J 401:39–47. doi:10.1042/BJ20061066
Lieberman I, Kornberg A, Simms ES (1955) Enzymatic synthesis of pyrimidine nucleotides; orotidine-5′-phosphate and uridine-5′-phosphate. J Biol Chem 215:403–451
Lucarelli AP et al (2010) Mycobacterium tuberculosis phosphoribosylpyrophosphate synthetase: biochemical features of a crucial enzyme for mycobacterial cell wall biosynthesis. PLoS One 5:e15494. doi:10.1371/journal.pone.0015494
McCoy AJ, Grosse-Kunstleve RW, Storoni LC, Read RJ (2005) Likelihood-enhanced fast translation functions. Acta Crystallogr D Biol Crystallogr 61:458–464. doi:10.1107/S0907444905001617
Newman J et al (2005) Towards rationalization of crystallization screening for small- to medium-sized academic laboratories: the PACT/JCSG+ strategy. Acta Crystallogr D Biol Crystallogr 61:1426–1431. doi:10.1107/S0907444905024984
Nygaard FB (2001) The molecular mechanism of catalysis and allosteric regulation in the phosphoribosyldiphosphate synthase from Bacillus subtilis. Thesis, University of Copenhagen
Otwinowski Z, Minor W (1997) Processing of X-ray diffraction data collected in oscillation mode. Methods Enzymol 276:307–326
Otwinowski Z, Minor W (2001) DENZO and SCALEPACK volume F: crystallography of biological macromolecules. International tables for crystallography. Springer, New York
Rasche ME, White RH (1998) Mechanism for the enzymatic formation of 4-(β-d-ribofuranosyl)aminobenzene 5′-phosphate during the biosynthesis of methanopterin. Biochemistry 37:11343–11351. doi:10.1021/bi973086q
Sharp PM, Li WH (1987) The codon adaptation index—a measure of directional synonymous codon usage bias, and its potential applications. Nucleic Acids Res 15:1281–1295
Smith PK et al (1985) Measurement of protein using bicinchoninic acid. Anal Biochem 150:76–85
Switzer RL (1969) Regulation and mechanism of phosphoribosylpyrophosphate synthetase. I. Purification and properties of the enzyme from Salmonella typhimurium. J Biol Chem 244:2854–2863
Taira M, Ishijima S, Kita K, Yamada K, Iizasa T, Tatibana M (1987) Nucleotide and deduced amino acid sequences of two distinct cDNAs for rat phosphoribosylpyrophosphate synthetase. J Biol Chem 262:14867–14870
White RH (1996) Biosynthesis of methanopterin. Biochemistry 35:3447–3456. doi:10.1021/bi952308m
Willemoës M, Hove-Jensen B, Larsen S (2000) Steady state kinetic model for the binding of substrates and allosteric effectors to Escherichia coli phosphoribosyl-diphosphate synthase. J Biol Chem 275:35408–35412
Xie G, Bonner CA, Jensen RA (2002) Dynamic diversity of the tryptophan pathway in chlamydiae: reductive evolution and a novel operon for tryptophan recapture. Genome Biol 3:research0051
Acknowledgments
We thank Qunxin She (University of Copenhagen) for kindly providing DNA of S. solfataricus, Tonny D. Hansen, Jens-Christian N. Poulsen and Dorthe Boelskifte for pertinent technical assistance. The MAXLAB and DANSCATT are gratefully acknowledged for providing the infrastructure facilitating collection of synchrotron data.
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical standards
The experiments performed in this study comply with the current laws of Denmark.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Communicated by S. Albers.
The atomic coordinates and structure factors have been deposited in the Protein Data Bank (PDB code 4TWB), Research Collaboratory for Structural Bioinformatics, Rutgers University, New Brunswick, NJ (http://www.rcsb.org).
Rights and permissions
About this article
Cite this article
Andersen, R.W., Leggio, L.L., Hove-Jensen, B. et al. Structure of dimeric, recombinant Sulfolobus solfataricus phosphoribosyl diphosphate synthase: a bent dimer defining the adenine specificity of the substrate ATP. Extremophiles 19, 407–415 (2015). https://doi.org/10.1007/s00792-014-0726-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00792-014-0726-x