Abstract
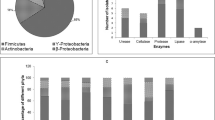
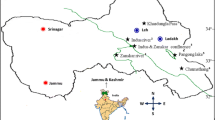
The Qinghai–Tibet Plateau represents a unique permafrost environment, being a result of high elevation caused by land uplift. And the urgency was that plateau permafrost was degrading rapidly under the current predicted climatic warming scenarios. Hence, the permafrost there was sampled to recover alkaliphilic bacteria populations. The viable bacteria on modified PYGV agar were varied between 102 and 105 CFU/g of dry soil. Forty-eight strains were gained from 18 samples. Through amplified ribosomal DNA restriction analysis (ARDRA) and phylogenetic analyses, these isolates fell into three categories: high G + C gram positive bacteria (82.3%), low G + C gram positive bacteria (7.2%), and gram negative α-proteobacteria (10.5%). The strains could grow at pH values ranging from 6.5 to 10.5 with optimum pH in the range of 9–9.5. Their growth temperatures were below 37°C and the optima ranging from 10 to 15°C. All strains grew well when NaCl concentration was below 15%. These results indicate that there are populations of nonhalophilic alkaliphilic psychrotolerant bacteria within the permafrost of the Qinhai-Tibet plateau. The abilities of many of the strains to produce extracellular protease, amylase and cellulase suggest that they might be of potential value for biotechnological exploitation.



Similar content being viewed by others
References
Bakermans C, Tsapin AI, Souza-Egipsy V, Gilichinsky DA, Nealson KH (2003) Reproduction and metabolism at −10°C of bacteria isolated from Siberian permafrost. Environ Microbiol 5(4):321–326
Becker FE, Volkmann CM (1961) A preliminary report on the bacteriology of permafrost in the Fairbanks area. Proc Alaskan Sci Conf 12:188
Boyd WL, Boyd JW (1964) The presence of bacteria in permafrost of the Alaskan arctic. Can J Microbiol 10:917–919
Cameron RE, Morelli FA (1974) Viable microorganisms from ancient Ross Island and Taylor Valley drill core. Antarct J US 9:113–116
Cheng G (1998) Glaciology and geocryology of China in the past 40 years: progress and prospect. J Glaciol Geocryol 20(3):213–226
Cole JR, Chai B, Marsh TL, Farris RJ, Wang Q, Kulam SA, Chandra S, McGarrell DM, Schmidt TM, Garrity GM, Tiedje JM (2003). The Ribosomal Database Project (RDP-II): previewing a new autoaligner that allows regular updates and the new prokaryotic taxonomy. Nucleic Acids Res 31:442–443
Felsenstein J (1985) Confidence limits on phylogenies: an approach using the bootstrap. Evolution 39:783–791
Friedmann EI (1994) Permafrost as microbial habitat. In: Gilinchisky DA (ed) Viable microorganisms in permafrost. Institute of Soil Science and Photosynthesis, Russian Academy of Science, Pushchino pp 21–26
Gilichinsky DA (2002) Permafrost model of extraterrestrial habitat. In Horneck G, Baumstark-Khan C (eds) Astrobiology: the quest for the conditions of life. pp 125–142
Gilichinsky DA, Wagener S (1995) Microbial life in permafrost: a historical review. Permafr Periglac Process 6:234–250
Gomori G (1955) Preparation of buffers. Methods Enzymol 1:138–146
Horikoshi K (1999) Alkaliphiles: some application of their products fro biotechnology. Microbiol Mol Biol Rev 63(4):735–750
Ito S, Kobayashi T, Ara K, Ozaki K, Kawai S, Hatada Y (1998) Alkaline detergent enzymes from alkaliphiles: enzymatic properties, genetics, and structures. Extremophiles 2:185–190
James N, Sutherland ML (1942) Are there living bacteria in permanently frozen subsoil? Can J Res Sect C Bot Sci 20(6):228–235
Jones EB, Grant WD, Collins NC, Mwatha WE (1994) Alkaliphiles: diversity and identification. In: Priest et al (eds) Bacterial diversity and systematics, Plenum, New York, pp 195–230
Jones EB, Grant WD, Duckworth AW, Owenson GG (1998) Microbial diversity of soda lakes. Extremophiles 2:191–200
Jukes TH, Cantor CR (1969) Evolution of protein molecules, In: Munro (ed) Mammalian protein metabolism. Academic, New York, pp 21–132
Junge K, Gosink JJ, Hoppe HG, Staley JT (1998) Arthrobacter, Brachybacterium and Planococcus isolates identified from antarctic sea ice brine. Description of Planococcus mcmeekinii, sp. nov. Syst Appl Microbiol 21(2):306–314
Khlebnikova GM, Gilichinsky DA, Fedorov-Davydov DC, Vorobyova EA (1990) Quantitative evaluation of microorganisms in permafrost deposits and buried soils. Microbiology 59:106–112
Khmelenina VN, Makutina VA, Kalyuzhnaya MG, Rivkina EM, Gilichinsky DA, Trotsenko Y (2002) Discovery of viable methanotrophic bacteria in permafrost sediments of northeast Siberia. Dokl Biol Sci 384:235–237
Krulwich TA (2000) Alkaliphilic prokaryotes. In: Dworkin et al (ed) The prokaryotes: an evolving electronic resource for the microbiological community, 2nd ed. Springer, Berlin
Kumar S, Tamura K, Jakobsen IB, Nei M (2001) MEGA2: molecular evolutionary genetics analysis software. Bioinformatics 12:1244–1245
Lane DJ (1991) 16S/23S rRNA sequencing. In: Stackebrandt E, Goodfellow M (eds) Nucleic acid techniques in bacterial systematics. Wiley, New York, pp 115–148
Li X, Cheng GD (1999) A GIS aided response model of high altitude permafrost to global change. Sci China Ser D 42(1):72–79
Lydolph MC, Jacobsen J, Arctander P, Gilbert MT, Gilichinsky DA, Hansen AJ, Willerslev E, Lange L (2005) Beringian paleoecology inferred from permafrost-preserved fungal DNA. Appl Environ Microbiol 71(2):1012–1017
Manafi M, Kneifel W (1990) Rapid methods for differentiating gram-positive from gram-negative aerobic and facultative anaerobic bacteria. J Appl Bacteriol 69:822–827
Michaud L, Di-Cello F, Brilli M, Fani R, Lo-Giudice A, Bruni V (2004) Biodiversity of cultivable psychrotrophic marine bacteria isolated from Terra Nova Bay. FEMS Microbiol Lett 230(1):63–71
Muller S (1943) Permafrost or permanently frozen ground and related engineering problems. Strategic Studies, 62. United States Army, Office Chief of Engineers, Military Intelligence Div. Strategic Eng. Study 62, pp 231
Niu F, Cheng G, Ni W, Jin D (2005) Engineering-related slope failure in permafrost regions of the Qinghai–Tibet Plateau. Cold Reg Sci Technol 42(3):215–225
Omelyansky VL (1911) Bakteriologicheskoe issledovanie Sanga mamonta Prilegayushchei pochvy Bacteriological investigation of the Sanga mammoth and surrounding soil. Arkhiv Biologicheskikh Nauk 16:335–340
Reddy GS, Prakash JS, Vairamani M, Prabhakar S, Matsumoto GI, Shivaji S (2002) Planococcus antarcticus and Planococcus psychrophilus spp. nov. isolated from cyanobacterial mat samples collected from ponds in Antarctica. Extremophiles 6(3):253–261
Rivkina E, Laurinavichius K, McGrath J, Tiedje J, Shcherbakova V, Gilichinsky D (2004) Microbial life in permafrost. Adv Space Res 33(8):1215–1221
Rivkina EM, Friedmann EI, McKay CP, Gilichinsky DA (2000) Metabolic activity of permafrost bacteria below the freezing point. Appl Environ Microbiol 66(8):3230–3233
Rothschild LJ, Mancinelli L (2001) Life in extreme environments. Nature 409:1092–1101
Ruger HJ, Fritze D, Sproer C (2000) New psychrophilic and psychrotolerant Bacillus marinus strains from tropical and polar deep-sea sediments and emended description of the species. Int J Syst Evol Microbiol 50:1305–1313
Saitou N, Nei M (1987) The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol 4:406–425
Sambrook J, Frisch EF, Maniatis T (1989) Molecular cloning: a laboratory manual, 2nd edn. Cold Spring Harbor Laboratory, Cold Spring Harbor
Sánchez-Porro C, Martín S, Mellado E, Ventosa A (2003) Diversity of moderately halophilic bacteria producing extracellular hydrolytic enzymes. J Appl Microbiol 94(2):295–300
Shi T, Reeves RH, Gilichinsky DA, Friedmann EI (1997) Characterization of viable bacteria from Siberian permafrost by 16S rDNA sequencing. Microb Ecol 33(3):169–179
Soina VS, Mulyukin AL, Demkina EV, Vorobyova EA, El-Registan GI (2004) The structure of resting bacterial populations in soil and subsoil permafrost. Astrobiology 4(3):345–358
Staley JT (1968) Prosthecomicrobium and Ancalomicrobium: new prosthecate freshwater bacteria. J. Bacteriol 95:1921–1942
Teather RM, Wood PJ (1982) Use of Congo red-polysaccharide interactions and in enumeration and characterization of cellulolytic bacteria from the bovine rumen. Appl Environ Microbiol 43:777–780
Thompson JD, Gibson TJ, Plewniak F, Jeanmougin F, Higgins DG (1997) The Clustal X Windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res 24:4876–4882
Tiago I, Chung AP, Verissimo (2004) A Bacterial diversity in a nonsaline alkaline environment: heterotrophic aerobic populations. Appl Environ Microbiol 70(12):7378–7387
Tiedje JM, Smith GB, Simkins S, Holben WE, Finney C, Gilichinsky DA (1994) Recovery of DNA, denitrifiers and patterns of antibiotic sensitivity in microorganisms from ancient permafrost soils of Eastern Siberia. In: Gilichinsky D (ed) Viable microorganisms in Permafrost. Russian Academy of Sciences, Pushchino, pp 83–98
Vishnivetskaya T, Kathariou S, McGrath J, Gilichinsky D, Tiedje JM (2000) Low-temperature recovery strategies for the isolation of bacteria from ancient permafrost sediments. Extremophiles 4(3):165–173
Vorobyova E, Soina V, Gorlenko M, Minkovskaya N, Zalinova N, Mamukelashvih A, Gilichinsky D, Rivkma E, Vishnivetskaya T (1997) The deep cold biosphere: facts and hypothesis. FEMS Microbiol Rev 20:277–290
Wang B, French HM (1995) Permafrost on the Tibet Plateau, China. Quat Sci Rev 14(3):255–274
Willerslev E, Hansen AJ, Binladen J, Brand TB, Gilbert MT, Shapiro B, Bunce M, Wiuf C, Gilichinsky DA, Cooper A (2003) Diverse plant and animalgenetic records from Holocene and Pleistocene sediments. Science 300:791–795
Williams PJ, Smith MW (1989) The frozen earth: fundamentals of geocryology. Cambridge University Press, Cambridge, pp. 306
Wu Q, Liu Y (2004) Ground temperature monitoring and its recent change in Qinghai–Tibet Plateau. Cold Reg Sci Technol 38:85–92
Zhu Y, Chen FH, Madsen D (2001) Early-holocene lake pollen record and environmental implication in Shiyanghe River drainage, arid region of China. Chin Sci Bull 46(19):1596–1602
Acknowledgments
This work was funded by Natural Science Foundation of China (30570270, 90102003, J0130084), China Postdoctoral Fund and State Key Lab of Frozen Soil and Engineering, CAREERI, CAS (SKLFSE200305). CAS Knowledge Innovation Key Directional Program (KZCX3-SW-339). The authors wish to thank Wei Liu and Peng Zhao for their help. We also are grateful to Managing Editor Prof Frank Robb and five anonymous referees for suggestive comments and modification.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by F. Robb.
Rights and permissions
About this article
Cite this article
Zhang, G., Ma, X., Niu, F. et al. Diversity and distribution of alkaliphilic psychrotolerant bacteria in the Qinghai–Tibet Plateau permafrost region. Extremophiles 11, 415–424 (2007). https://doi.org/10.1007/s00792-006-0055-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00792-006-0055-9