Abstract
Objective
This study investigated the biological activity of the essential oil from Cymbopogon nardus and of the phytoconstituent citronellal on Candida strains as to the inhibition of adherence to dental implants and cover screws.
Material and methods
The essential oil was analyzed by gas chromatography coupled to mass spectrometry (GC-MS) and had its MIC and MFC determined against 12 strains of Candida. Then, tests of inhibition of adherence to the dental implants and cover screws were carried out using the MIC of the substances, followed by scanning electron microscopy analysis. Nystatin and chlorhexidine were used as positive controls, and experiments were performed in triplicate.
Results
The analysis by GC-MS of the essential oil identified citronellal as the major compound. The MICs of the essential oil, citronellal, chlorhexidine, and nystatin—able to inhibit 100 % of the strains—were found to be 64, 512, 64, and 32 μg/ml, respectively. The essential oil significantly inhibited the adherence of Candida albicans to the dental implants and cover screws (p < 0.001). Citronellal inhibited yeast adherence only to the dental implants (p < 0.001), and no significant results were found for the cover screws (p > 0.05) compared to the growth control.
Conclusion
The essential oil and citronellal have proven antifungal activity and are able to inhibit the in vitro adherence of C. albicans.
Clinical relevance: There has been a search for alternative natural product-containing formulations that should be effective in inhibiting adherence of yeasts to the surfaces of materials and also able to treat oral fungal infections. Further trials could make these products an alternative to chemical removal of peri-implant biofilm.
Similar content being viewed by others
Introduction
The use of dental implants has become an alternative in the rehabilitation of edentulous patients, with a success rate of approximately 96 %. In spite of that, a considerable number of implant failures occurs throughout the years [1]. The causes of the failures may be related to endogenous and exogenous factors. Endogenous factors include those affecting the patient’s general health and oral hygiene habits, which can trigger peri-implant infections known as peri-implantitis. Exogenous factors, in turn, are related to the type of material used in the manufacture of implants, biocompatibility, surface features, and implant design [2, 3].
Peri-implantitis is defined as an inflammation of the mucosa with the presence of suppuration, bleeding, and continuous bone loss around the implants [4]. Risk factors for peri-implantitis include early exposure of implants and poor oral hygiene, resulting in accumulation of biofilm [5] that can be initiated on the surface of the implant as soon as it is exposed in the oral cavity [6, 7]. The microbiota found in the peri-implant disease is similar to that found insites with gingivitis and advanced periodontitis [8]. In addition to periodontopathogenic bacteria, peri-implant disease includes microorganisms of the resident microbiota of the oral cavity, such as Candida spp., which are capable of damaging the tissue due to proteinase production, enhance formation of diverse and potentially pathogenic biofilms, besides modulating the host immune response, which in the case of peri-implant infection, promotes tissue destruction, particularly the activation of osteoclasts [9–11].
In general, bacterial and/or fungal biofilms have greater resistance to host defenses and to conventional antimicrobial therapy, resulting in serious and persistent infections [12, 13]. The adherence of microorganisms to titanium surfaces has already been described in vivo and in vitro; however, there is little information in the literature about the inhibition of yeast adherence to the surfaces of dental implants and cover screws, even considering that these devices provide the substrate necessary for formation of fungal biofilms and thus can serve as a reservoir for infection/reinfection by Candida albicans [14].
C. albicans is a commensal and opportunistic pathogen that causes infections usually as a result of a change in the host immune response. Its virulence is attributed to morphological plasticity and ability to form biofilms [15]. The early and essential step in the pathogenesis of oral candidiasis involves the attachment of C. albicans to a receiving surface or implanted device, such as prostheses and dental implants [12, 13]. In general, yeast cells have a high potential for adherence to artificial materials, in much the same way as to the oral tissues [16].
Certain factors are essential to make the mechanical control of biofilm successful, for instance, frequency, technique, and brushing time, in addition to patient’s motivation and performance. Some studies [7–9, 17] point out that the use of antimicrobials (systemic, local, or combined) is beneficial in the treatment of peri-implant infections; however, there is not a specific protocol so far, but only proposals addressing the antimicrobial treatment of peri-implantitis.
Chlorhexidine is proven to act upon biofilm formation with a broad antimicrobial spectrum against both bacteria and fungi [18]. As such, it is indicated as adjunctive chemical method for the treatment of peri-implantitis [19–21]. However, adverse effects of chlorhexidine, such as unpleasant taste, taste changes, pigmentation of teeth, and oral desquamation, are factors limiting its prolonged use [22, 23].
New agents have been proposed in order to minimize unwanted reactions presented by users with the use of antimicrobials, as well as increased resistance by microorganisms. Microbial resistance levels have been increasing due to the indiscriminate use of antimicrobials, which is driving researchers to study new antimicrobial substances from various sources, including medicinal plants [24]. Medicinal plants play an important role in global health and continue to be used despite the great advances observed in modern medicine. It is estimated that 25–30 % of all drugs considered as therapeutic agents are derived from natural products [25].
Among the substances obtained from plants, the essential oils stand out as natural, complex compounds characterized by strong odor and volatility. They are produced by aromatic plants as secondary metabolites and have about two or three major compounds at relatively high concentrations (20–70 %) [26]. Thus, the present study aimed to evaluate the inhibition of adherence of C. albicans to dental implants and cover screws by Cymbopogon nardus essential oil and citronellal.
Materials and methods
Microbiological tests were performed in the Mycology Laboratory of the Department of Pharmaceutical Sciences, Center for Health Sciences, and scanning electron microscopy analysis was performed at the Technology Center, Federal University of Paraíba (UFPB).
The dental implants DSP BIOMEDICAL® OSTEOFIT HE 4.1 × 11.5 mm SLA ZIRCON® and the cover screws DSP BIOMEDICAL® were used in this study, as well as C. nardus essential oil (QUINARI®) and the phytoconstituent citronellal (SIGMA-ALDRICHT®). In order to investigate the biological activity of the products, the following clinical and collection strains were used: C. albicans (ATCC 76485); C. albicans (ATCC 76645); C. albicans (LM P20); C. albicans (LM 62); C. albicans (LM 122); C. albicans (LM 108); C. albicans (LM 86); C. albicans (LM 111); C. tropicalis (LM 45); C. tropicalis (ATCC 13803); C. tropicalis (LM 14); and C. tropicalis (LM 20).
Nystatin (SIGMA-ALDRICHT®) and chlorhexidine (RIOQUÍMICA®) were used as positive controls in the tests of antimicrobial susceptibility. The culture media used were RPMI-1640 (HIMEDIA®), Sabouraud Dextrose Broth (SDB), and Sabouraud Dextrose Agar (SDA) (DIFCO®), which were prepared according to the manufacturers’recommendation.
For the preparation of the inoculum, the selected strain was maintained in SDA for 24–48 h at 35 °C and subsequently standardized in sterile saline solution using a suspension of barium sulfate n. 0.5 of the McFarland scale. After being stirred with the aid of a Vortex device (FANEM) for 2 min, the suspension was adjusted to 90 % transmittance on a spectrophotometer (LEITZ-PHOTOMETER 340-800) to contain approximately 106 CFU/ml [27–29].
Chromatography and mass spectrometry
The compounds were identified using a gas chromatograph coupled to a mass spectrometer (SHIMADZU GC-MS-QP5050A) with capillary column (J & W SCIENTIFIC®) and a stationary phase of 5 % phenyl and 95 % dimethylpolysiloxane measuring 30-m length, 0.25-mm inner diameter, and 0.25-mm film thick. The initial temperature program was 60 to 240 °C (3 °C/min). The programming time of the run was 60 min, and the injector oven temperature was 250 °C. Helium was used as carrier gas (mobile phase) at a flow rate of 1.0 ml/min with a 1:20 split ratio and injection volume of 1 μl.
The ionization of the components was performed by electron impact at 70 eV, using a 1.25-kV detector. The spectrometer was operated in SCAN mode, scanning a range of masses from 40 to 500 atomic mass unit (a.m.u). The ion source temperature was 250 °C, and the compounds were identified by comparing their mass spectra with those existing in the equipment database (MIST library, 2008).
The essential oil sample was injected at a concentration of 2 ppm, and hexane was used as solvent. Analyses of the chromatogram and mass spectra were performed using the equipment library, and the integration parameters used were width, 3, and slope, 2000.
Minimum inhibitory concentration (MIC)
The determination of MIC of the essential oil, phytoconstituent, nystatin, and chlorhexidine was performed by the microdilution technique [27, 28]. Initially, 100 μl of RPMI-1640 culture medium was added to the wells of the plate. Then, 100 μl of the experimental substances was also added to the wells at the concentration of 1024 μg/ml, and both the medium and the sample were homogenized. Subsequently, the sample was serially diluted by transferring an aliquot of 100 μl from the first well to the following ones. The concentrations ranged from 1024 to 8 μg/ml. Aliquots of 10 μl of the inoculum were dispensed into the wells of each column. In parallel, controls of strain viability and susceptibility to nystatin and chlorhexidine were also carried out using the same technique. Tests were performed in triplicate, and plates were incubated at 35 °C for 24–48 h. The reading for MIC determination was performed by the visual method, taking into consideration whether or not there was formation of cell clusters at the bottom of the wells. As such, MIC was considered as the lowest concentration of the test product capable of producing visible growth inhibition of yeast strains used in microbiological assays [30].
Minimum fungicidal concentration (MFC)
After determining the MIC, the content of the wells corresponding to the inhibitory and two higher concentrations (MIC, MICx2, and MICx4) as well as that of the positive controls was subcultured on SDA plates. The reading of MFC was based on the growth of the controls after 24–48 h of incubation at 35 °C. MFC was considered as the lowest concentration of the test product that hampered visible growth in the subculture. Tests were performed in triplicate [31].
Inhibition of fungal adherence to dental implants and cover screws
The strain of C. albicans (ATCC 76645) was used in the test of inhibition of adherence, performed in triplicate. This fungal species is known for its virulence associated with morphological plasticity and ability to form biofilms on different substrates. Furthermore, the use of an ATCC reference strain enables the reproducibility of the experimental conditions and comparison with other studies. The strain was maintained in SDA, incubated at 35 °C for 24–48 h. The implants and cover screws were placed individually into glass vials containing 1.9 ml of SDB plus the product to be tested in a sufficient amount capable of providing a final concentration corresponding to the MIC. A total of 100 μl of yeast suspension (106 CFU/ml) were also added, and the tubes were incubated at 35 °C for 48 h. Both the implants (n = 3) and cover screws (n = 3) were randomly allocated into five groups: (A) growth control; (B) C. nardus; (C) citronellal; (D) nystatin; and (E) chlorhexidine, totalizing 15 dental implants and 15 cover screws.
After the incubation period (48 h), the materials were washed with 1 ml of sterile distilled water and placed in sterile vials containing 5 ml of saline solution (0.85 % NaCl). Then, the vials were stirred in a Vortex apparatus for 60 s. After this procedure, 10 μl of the solution was seeded on Petri dishes containing SDA, which were incubated at 35 °C for 48 h. Ultimately, the counting of CFU/ml was made [32].
Scanning electron microscopy (SEM) analysis
In order to conduct the SEM analysis, baseline micrographs of the surfaces of one dental implant and one cover screw were taken prior to incubation of the microorganisms. To allow the adherence, five dental implants and five cover screws were individually placed in sterile vials containing 1 ml of SDB, 0.1 ml of yeast suspension (106 CFU/ml), and 1.0 ml of the test products at MIC (groups B, C, D, and E). Then, the vials were incubated at 35 °C for 24–48 h. In group (A), the vials contained only 1.0 ml of SDB and 0.1 ml of yeast suspension (106 CFU/ml).
After the incubation period, the materials were removed from the vials and underwent metallization with gold plasma (Emitech K550x®). Posteriorly, microbial adherence was visualized under a scanning electron microscope (LEO 1430®).
Statistical analysis
The data were analyzed on the software GraphPad Prism 4. Analysis of variance (ANOVA) with Tukey’s posttest was carried out, with a confidence interval of 95 %.
Results
Chromatographic profile and identification of compounds
The analysis by gas chromatography couple to mass spectrometry (GC-MS) allowed the characterization of C. nardus essential oil and identification of compounds based on information of the retention time and molecular weight. Additionally, the compounds were quantified according to the peak areas (Table 1). The data were compared to the library available in the chromatographer.
The terpenoid citronellal was found to be the major compound (37.75 %), followed by geraniol (18.84 %), and citronellol (14.27 %).
Minimum inhibitory concentration (MIC) and minimum fungicidal concentration (MFC)
The products evaluated were able to inhibit the growth of all Candida strains tested herein. C. nardus essential oil inhibited 75 % (MIC75% = 32 μg/ml) of the strains at a concentration of 32 μg/ml, although C. albicans ATCC 76485, C. albicans LM 108, and C. albicans LM 86 were only susceptible at the concentration of 64 μg/ml (Table 2). This essential oil had MFC66.6% of 32 μg/ml, and the strains of C. albicans ATCC 76485 and C. albicans LM 62 were found to be more resistant (MFC of 128 μg/ml).
Citronellal presented MIC75% and MFC58.3% of 256 μg/ml, although MIC and MFC values ranged between 16 and 512 μg/ml and 16 and 1024 μg/ml, respectively. C. albicans LM 122 and C. albicans LM 86 were found to be among the most resistant strains. Chlorhexidine at the concentrations between 8 and 64 μg/ml was able to inhibit all strains, with MFC58.3% of 64 μg/ml.
Lower values of MIC and MFC were observed for nystatin, which had MIC75% of 8 μg/ml and MFC75% of 16 μg/ml. C. albicans ATCC 76485 and C. tropicalis LM 14 showed higher resistance to nystatin, with MFC of 128 and 64 μg/ml, respectively.
Inhibition of fungal adherence to the dental implants and cover screws
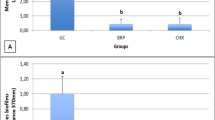
The test of inhibition of adherence to the dental implants (Fig. 1) indicated that the experimental substances evaluated, as well as nystatin and chlorhexidine, were able to inhibit fungal growth, with a reduction in the number of colony-forming units (CFU). All substances showed statistically significant results compared to the growth control (p < 0.001), but with no statistical difference when compared between each other (p > 0.05). As to the inhibition of adherence to the cover screws (Fig. 2), C. nardus essential oil, nystatin, and chlorhexidine also showed statistically significant results when compared to the growth control (p < 0.001), with no significant difference when compared between each other (p > 0.05). Citronellal was not effective in inhibiting adherence to the cover screws, with results similar to those of the growth control.
Scanning electron microscopy (SEM) analysis
The SEM analysis was performed in order to obtain images of the surfaces of the dental implants and cover screws after treatment with the experimental substances at MIC. Firstly, a micrograph of the surfaces of the implant (Fig. 3a) and cover screw (Fig. 4a) was taken to allow the visualization of the surfaces with no microorganisms. Then, micrographs of the dental implants (Fig. 3b) and cover screws (Fig. 4b) were taken to show C. albicans adherence to the surfaces. The figures 5 and 6 reveal absence of yeast adherence to the surfaces after treatment with the antifungal agents tested.
Discussion
The interest in studying the anti-Candida activity of C. nardus essential oil and citronellal is based on their proven activity against fungal species [33, 34] and on their notable low toxicity on human epithelial cells. A previous study [35] showed that these products allow 75 % cell viability at the concentration of 75 μg/ml, which is much higher than that proposed in our study.
The analysis of C. nardus essential oil by GC-MS identified the terpenoid citronellal as the major compound (37.75 %). This finding is in agreement with other studies in the literature [35–38], in which citronellal was quantified ranging from 29.6 to 35.9 %. A number of factors may influence the chemical composition of the essential oil. The most important ones include the following: the plant part used for obtaining the oil, its origin, development stage, and climatic conditions [39].
The results of this study showed that C. nardus essential oil had lower values of MIC and MFC in 83.3 % of the tested strains when compared to citronellal. This fact may be due to the existing synergism between the compounds of the essential oil, which makes it to display a better biological activity [40].
Even though some studies have shown that C. nardus essential oil as well as its major constituent citronellal have antifungal properties [34, 41], their biological activity against Candida species remains poorly studied. Furthermore, little information is known about their antibacterial activity, particularly against oral pathogens, even though the essential (whose major compound is citronellal) was found to be active against periodontopathogenic bacteria [42].
The present study demonstrated that the essential oil and the phytoconstituent citronellal were able to inhibit 100 % of the strains at the concentrations of 64 and 512 μg/ml, respectively. Investigating the antifungal activity of C. nardus essential oil on C. albicans, another study [43] found MIC of 450 μg/ml. These differences in the MIC values can be attributed to the chemical composition of the essential oil and to the different strains of microorganisms, although belonging to the same species.
The essential oil also had lower MIC and MFC values in relation to chlorhexidine against most of the strains studied. Chlorhexidine has proven antimicrobial action upon biofilm formation. However, adverse effects such as unpleasant taste, taste changes, pigmentation of teeth, and oral desquamation are limiting factors for its prolonged use [22, 23].
Although nystatin had the lowest MIC and MFC values against most of the strains tested, some authors [44, 45] showed that Candida species may exhibit resistance to this standard antifungal. This resistance can be caused by the indiscriminate use of antimicrobials, which makes it interesting to search for new substances, mainly of natural origin. Therefore, further in vitro and in vivo studies of C. nardus essential oil and citronellal should be carried out, in view of the utmost importance of elucidating issues like safety, tolerability, and possible adverse effects related to the use of these chemical agents.
In general, essential oils act by breaking or disrupting membranes due to their lipophilic compounds, which leads to loss of several enzymes and nutrients through the cell membrane [46, 47]. In the test of inhibition of adherence to the dental implants, C. nardus essential oil and the phytoconstituent citronellal were able to inhibit fungal growth and showed statistically significant results compared to the growth control (p < 0.001), with no statistical difference when compared to nystatin and chlorhexidine (p > 0.05) though. As to the inhibition of adherence to the cover screws, C. nardus essential oil, nystatin, and chlorhexidine inhibited the growth of C. albicans with statistically significant results compared to the growth control (p < 0.001). However, no significant difference was found when these groups were compared between each other (p > 0.05). Citronellal was not effective in inhibiting fungal adherence, displaying results similar to those of the growth control. C. nardus essential oil showed better results compared to citronellal likely due to the synergism between the compounds present in the oil, leading to a better biological activity.
In the qualitative analysis by SEM, C. nardus essential oil and citronellal were able to inhibit the adherence of the tested strains to the surface of the dental implants and cover screws. This study demonstrated by SEM analysis that C. albicans was able to adhere to the surfaces of dental implants and cover screws, corroborating other reports [10, 12, 13, 48, 49] on the ability of this species in adhering to surfaces and forming biofilms thereon. The dental implants used in this study had undergone surface treatment, which favors cell adherence and accelerates the process of osseointegration in the rehabilitation of edentulous patients [50]. It is worth noting that the SEM analyses were based on a qualitative methodological approach complementary to the findings of the microbiological assays.
In general, dental implants have roughness that are, on average, greater than 2 μm [51, 52], which provides an effective adherence of C. albicans [53, 54] and leads the biofilm to be more resistant to chemical agents [13].
Conclusion
The essential oil from C. nardus showed antifungal activity and was able to inhibit the adherence of C. albicans to the surfaces of the materials assayed, similarly to nystatin and chlorhexidine (standard drugs). Nevertheless, further in vitro and in vivo research should be undertaken to investigate the possible mechanisms of action and toxicological effects, with the purpose of developing an effective natural product-containing formulation able to inhibit fungal adherence and infections.
References
Roos-Jansaker AM, Lindahl C, Renvert H, Renvert S (2006) Nine-to fourteen-year follow-up of implant treatment. Part II: presence of peri-implant lesions. J Clin Periodontol 33:290–295
Schwartz-Arad D, Samet N, Samet N, Mamlider A (2002) Smoking and complications of endosseous dental implants. J Periodontol 73(2):153–157
Van Steenberghe D, Jacobs R, Desnyder M, Maffei G, Quirynen M (2002) The relative impact of local and endogenous patient-related. Clin Oral Implants Res 13(6):617–622
Heitz-Mayfield LJA (2008) Peri-implant diseases: diagnosis and risk indicators. J Clin Periodontol 35(suppl 8):293–304
Lindhe J, Meyle J (2008) Peri-implant diseases: consensus report of the sixth European workshop on periodontology. J Clin Periodontol 35(suppl8):282–285
Klinge B, Hultin M, Berglundh T (2005) Peri-implantitis. Dent Clin North Am 49:661–676
Humphrey S (2006) Implant maintenance. Dent Clin North Am 50(3):463–478
Lang NP, Wilson TG, Corbet EF (2000) Biological complications with dental implants: their prevention, diagnosis and treatment. Clin Oral lmplants Res 11(suppl 1):146–155
Pye AD, Lockhart DEA, Dawson MP, Murray CA, Smith AJ (2009) A review of dental implants and infection. J Hosp Infect 72(2):104–110
Burgers R, Hahnel S, Reichert TE, Rosentritt M, Behr M, Gerlach T, Handel G, Gosau M (2010) Adhesion of Candida albicans to various dental implant surfaces and the influence of salivary pellicle proteins. Acta Biomater 6:2307–2313
Villard N, Seneviratne C, Tsoi JKH, Heinonen M, Matinlinna J (2015) Candida albicans aspects of novel silane system-coated titanium and zirconia implant surfaces. Clin Oral Implants Res 26(3):332–341
Ramage G, Martinez JP, Lopez-Ribot JL (2006) Candida biofilms on implanted biomaterials: a clinically significant problem. FEMS Yeast Res 6:979–986
Tsang CSP, Ng H, Mc Millan AS (2007) Antifungal susceptibility of Candida albicans biofilms on titanium discs with different surface roughness. Clin Oral Invest 11:361–368
Leonhardt A, Bergstrom C, Lekholm U (2003) Microbiologic diagnostics at titanium implants. Clin Implant Dent Relat Res 5:226–232
Monge RA, Roma’n E, Nombela C, Pla J (2006) The MAP Kinase signal transduction network in Candida albicans. Microbiology 152(1):905–912
Blankenship JR, Mitchell AP (2006) How to build a biofilm: a fungal perspective. Curr Opin Microbiol 9:588–594
Triplett RG, Andrews JA, Hallmon WW (2003) Management of peri-implantitis. Oral Maxillofac Surg Clin North Am 15(1):129–138
Menendez A, Li F, Michalek SM, Kirk K, Makhija SK, Childers NK (2005) Comparative analysis of the antibacterial effects of combined mouthrinses on Streptococcus mutans. Oral Microbiol Immunol 20:31–34
Renvert S, Roos-Jansaker AM, Claffey N (2008) Non-surgical treatment of peri-implant mucositis and peri-implantitis: a literature review. J Clin Periodontol 35:305–315
Heitz-Mayfield LJ, Salvi GE, Mombelli A, Faddy M, Lang NP (2012) Anti-infective surgical therapy of peri-implantitis. A 12-month prospective clinical study. Clin Oral Implants Res 23:205–210
Wiltfang J, Zernial O, Behrens E, Schlegel A, Warnke PH, Becker ST (2012) Regenerative treatment of peri-implantitis bone defects with a combination of autologous bone and a demineralized xenogenic bone graft: a series of 36 defects. Clin Implant Dent Relat Res 14:421–427
Paraskevas S (2005) Randomized controlled clinical trials on agents used for chemical plaque control. Int J Dent Hyg 3(4):162–178
Gunsolley JC (2006) A meta-analysis of six-month studies of antiplaque and antigingivitis agents. J Am Dent Assoc 137:1649–1657
Bansod S, Rai M (2008) Antifungal activity of essential oils from Indian medicinal plants against human pathogenic Aspergillus fumigates and A. niger. World J Med Sci 3(2):81–88
Calixto JB (2005) Twenty-five years of research on medicinal plants in Latin America: a personal review. J Ethnopharmacol 100:131–134
Bakkali F, Averbeck S, Averbeck D, Idaomar M (2008) Biological effects of essential oils—a review. Food Chem Toxicol 46:446–475
Cleeland R, Squires E (1991) Evaluation of new antimicrobials in vitro and in experimental animal infections In: Lorian VAntibiotics in laboratory medicine. 3rd edn. Baltimore: Williams and Wilkiam, pp 739–787
Hadacek F, Greger H (2000) Testing of antifungal natural products: methodologies, comparability of results and assay choice. Phytochem Anal 11:137–147
Bauer AWMM, Kirby JC, Turck M (1966) Antibiotic susceptibility testing by astandardized single disk method. Am J Clin Pathol 45(3):493–496
Deswal DP, Chand U (1997) Standartization of the tetrazolium test for viability estimation in ricebean (Vigna umbellate T.) seeds. Seed Sci Technol 25:409–417
Ernst ME, Klepser ME, Wolfe EJ, Pfaller MA (1996) Antifungal Dynamics of LY 303366, an investigational echinocandin B analog, against Candida spp. Diagn Micr Infec Dis 26:125–131
Carreto CFP, Navas EAFA, Paradella TC, Oliveira LD, Junqueira JC, Jorge AOC (2007) Efeitos do chá de tomilho sobre a aderência in vitro de Streptococcusmutansao esmalte dentário e Candida albicansà resina acrílica. Rev Odontol UNESP 36(3):281–186
Billerbeck VG, Roques CG, Bessière JM, Fonvielle JL, Dargent R (2001) Effects of Cymbopogonnardus (L.) W. Watson essential oil on the growth and morphogenesis of Aspergillusniger. Can J Microbiol 47(1):9–17
Zore GB, Thakre AD, Jadhav S, Karuppayil SM (2011) Terpenoids inhibit Candida albicans growth by affecting membrane integrity and arrest of cell cycle. Phytomedicine 18:1181–1190
Koba K, Sanda K, Guyon C, Raynaud C, Chaumont JP, Nicod L (2009) In vitro cytotoxic activity of CymbopogoncitratusL. and CymbopogonnardusL. essential oils from Togo. Bangladesh J Pharmacol 4:29–34
Kpoviessi S, Bero J, Agbani P, Gbaguidi F, Kpadonou-Kpoviessi B, Sinsin B, Accrombessi G, FrédérichM MM, Quetin-Leclercq J (2014) Chemical composition, cytotoxicity and in vitroantitrypanosomal and antiplasmodial activity of the essential oils of four Cymbopogonspecies from Benin. J Ethnopharmacol 151:652–659
Wei LS, Wee W (2013) Chemical composition and antimicrobial activity of Cymbopogonnarduscitronella essential oil against systemic bacteria of aquatic animals. Iran J Microbiol 5(2):147–152
Roszaini K, NorAzah MA, Mailina J, Zaini S, Faridz ZM (2013) Toxicity and antitermite activity of the essential oils from Cinnamomumcamphora, Cymbopogonnardus, Melaleucacajuputiand Dipterocarpussp. against Coptotermescurvignathus. Wood Sci Technol 47:1273–1284
Ozcan M, Erkmen O (2001) Antimicrobial activity of the essential oils of Turkish plant spices. Eur Food Res Technol 212(6):658–660
Williamson EM (2001) Synergy and other interactions in phytomedicines. Phytomedicine 8(5):401–409
Li WR, Shi QS, Ouyang YS, Chen YB, Duan SS (2013) Antifungal effects of citronela oil against Aspergillusniger ATCC 16404. Appl Microbiol Biotechnol 97(16):7483–7492
Wongsariya K, Phanthong P, Bunyapraphatsara N, Srisukh V, Chomnawang MT (2014) Synergistic interaction and mode of action of Citrus hystrix essential oil against bacterial causing periodontal diseases. Pharm Biol 52(3):273–280
Corrêa NB, Gonçalves GMS (2010) Atividade antifúngica de óleos essenciais de plantas aromáticas frente a diferentes espécies de Candida. Anais do XV encontro de iniciação científica da PUC-Campinas
Chandra J, Mukherjee PK, Leidich SD, Faddoul FF, Hoyer LL, Douglas LJ, Ghannoum MA (2001) Antifungal resistance of candidal biofilms formed on denture acrylic in vitro. J Dent Res 80:903–908
Kuhn DM, George T, Chandra J, Mukherjee PK, Ghannoum MA (2002) Antifungal susceptibility of Candida biofilms: unique efficacy of amphotericin B lipid formulations and echinocandins. Antimicrob Agents Chemother 46:1773–1780
Cowan MM (1999) Plant products as antimicrobial agents. Clin Microbiol Rev 12:564–582
Cox SD, Mann CM, Markham JL, Bell HC, Gustafson JE, Warmington JR, Wyllic SG (2000) The mode of antimicrobial action of essential oils of Melaleucaalternifolia(tea tree oil). J Appl Microbiol 88:170–175
Harder S, Podschun R, Grancicova L, Mehl C, Kern M (2013) Analysis of the intraimplantmicroflora of two-piece dental implants. Clin Oral Invest 17:1135–1142
Shibli JA, Martins MC, Lotufo RFM, Marcantonio E (2003) Microbiologic and radiographic analysis of ligature-induced peri-implantitis with diferente dental implant surfaces. Int J Oral Maxillofac Implants 18(3):383–390
Nasatzky E, Gultchin J, Schwartz Z (2003) The role of surface roughness in promoting osteointegration. Refuat Hapeh Vehashinayim 20(3):8–19
Wennerberg A, Albrektsson T (2000) Suggested guidelines for the topographic evaluation of implant surfaces. Int J Oral Maxillofac Implants 15:331–344
Novaes AB Jr, Papalexiou V, Grisi MF, Souza SS, Taba M Jr, Kajiwara JK (2004) Influence of implant microstructure on the osseointegration of immediate implants placed in periodontally infected sites. A histomorphometric study in dogs. Clin Oral Implants Res 15:34–43
Mimica LMJ, Ueda SMY, Martino MDV, Navarini A, Martini IJ (2009) Candida infection diagnosis: evaluation of Candida species identification and characterization of susceptibility profile. J Bras Pat Med Lab 5(1):17–23
Quirynen M, Van Der Mei HC, Bollen CM, Schotte A, Marechal M, Doornbusch GI, NAERT I, Busscher HJ, Van Steenberghe D (1993) An in vivo study of the influence of the surface roughness of implants on the microbiology of supra- and subgingival plaque. J Dent Res 72(9):1304–1309
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Trindade, L.A., de Araújo Oliveira, J., de Castro, R.D. et al. Inhibition of adherence of C. albicans to dental implants and cover screws by Cymbopogon nardus essential oil and citronellal. Clin Oral Invest 19, 2223–2231 (2015). https://doi.org/10.1007/s00784-015-1450-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00784-015-1450-3