Abstract
Background
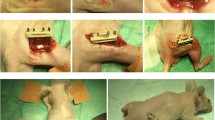
In the last few years, several attempts have been made to treat large bone loss, including the use of tissue engineering with osteoinductive scaffolds and cells. This study highlights the role of mesenchymal stem cells from adipose tissue (ASCs; adipose-derived stem cells) in a rabbit bone regeneration model.
Methods
We compared the neoformed bone tissues achieved by treating critical tibial defects with either hydroxyapatite alone (HA, group I) or hydroxyapatite–autologous ASC constructs (ASCs-HA, group II), investigating their histomorphometric, immunohistochemical and biomechanical properties.
Results
After eight weeks of follow-up, we observed advanced maturation and a spatial distribution of new bone that was more homogeneous in the inner parts of the pores in group II, not just along the walls (as seen in group I). The new tissue expressed osteogenic markers, and biomechanical tests suggested that the newly formed bone in group II had a higher mineral content than that in group I. Although variability in differentiation was observed among the different cell populations in vitro, no differences in bone healing were observed in vivo; the variability seen in vitro was probably due to local microenvironment effects.
Conclusions
Tibial defects treated with rabbit ASCs-HA showed an improved healing process when compared to the process that occurred when only the scaffold was used. We suggest that implanted ASCs ameliorate the bone reparative process either directly or by recruiting resident progenitor cells.





Similar content being viewed by others
References
Sasso RC, Williams JI, Dimasi N, Meyer PR Jr. Postoperative drains at the donor sites of iliac-crest bone grafts. A prospective, randomized study of morbidity at the donor site in patients who had a traumatic injury of the spine. J Bone Joint Surg Am. 1998;80:631–5.
Muschler GF, Raut VP, Patterson TE, Wenke JC, Hollinger JO. The design and use of animal models for translational research in bone tissue engineering and regenerative medicine. Tissue Eng Part B Rev. 2010;16:123–45.
Lee MJ, Sohn SK, Kim KT, Kim CH, Ahn HB, Rho MS, Jeong MH, Sun SK. Effect of hydroxyapatite on bone integration in a rabbit tibial defect model. Clin Orthop Surg. 2010;2:90–7.
Viateau V, Guillemin G, Bousson V, Oudina K, Hannouche D, Sedel L, Logeart-Avramoglou D, Petite H. Long-bone critical-size defects treated with tissue-engineered grafts: a study on sheep. J Orthop Res. 2007;25:741–9.
Xiao C, Zhou H, Ge S, Tang T, Hou H, Luo M, Fan X. Repair of orbital wall defects using biocoral scaffolds combined with bone marrow stem cells enhanced by human bone morphogenetic protein-2 in a canine model. Int J Mol Med. 2010;26:517–25.
de Girolamo L, Arrigoni E, Stanco D, Lopa S, Di Giancamillo A, Addis A, Borgonovo S, Dellavia C, Domeneghini C, Brini AT. Role of autologous rabbit adipose-derived stem cells in the early phases of the repairing process of critical bone defects. J Orthop Res. 2011;29:100–8.
de Girolamo L, Sartori MF, Arrigoni E, Rimondini L, Albisetti W, Weinstein RL, Brini AT. Human adipose-derived stem cells as future tools in tissue regeneration: osteogenic differentiation and cell-scaffold interaction. Int J Artif Organs. 2008;31:467–79.
de Girolamo L, Lopa S, Arrigoni E, Sartori MF, Baruffaldi Preis FW, Brini AT. Human adipose-derived stem cells isolated from young and elderly women: their differentiation potential and scaffold interaction during in vitro osteoblastic differentiation. Cytotherapy. 2009;11:793–803.
Tapp H, Hanley EN Jr, Patt JC, Gruber HE. Adipose-derived stem cells: characterization and current application in orthopaedic tissue repair. Exp Biol Med (Maywood). 2009;234:1–9.
Thesleff T, Lehtimäki K, Niskakangas T, Mannerström B, Miettinen S, Suuronen R, Öhman J. Cranioplasty with adipose-derived stem cells and biomaterial: a novel method for cranial reconstruction. Neurosurgery. 2011;68:1535–40.
Wu L, Cai X, Zhang S, Karperien M, Lin Y. Regeneration of articular cartilage by adipose tissue derived mesenchymal stem cells: perspectives from stem cell biology and molecular medicine. J Cell Physiol. 2012;. doi:10.1002/jcp.24255.
Arrigoni E, Lopa S, de Girolamo L, Stanco D, Brini AT. Isolation, characterization and osteogenic differentiation of adipose-derived stem cells: from small to large animal models. Cell Tissue Res. 2009;338:401–11.
Bosetti M, Zanardi L, Hench L, Cannas M. Type I collagen production by osteoblast-like cells cultured in contact with different bioactive glasses. J Biomed Mater Res A. 2003;64:189–95.
Donath K, Breuner G. A method for the study of undecalcified bones and teeth with attached soft tissues. The Sage–Schliff (sawing and grinding) technique. J Oral Pathol. 1982;11:318–26.
Paietta RC, Campbell SE, Ferguson VL. Influences of spherical tip radius, contact depth, and contact area on nanoindentation properties of bone. J Biomech. 2011;44:285-90.
Hengsberger S, Kulik A, Zysset P. Nanoindentation discriminates the elastic properties of individual human bone lamellae under dry and physiological conditions. Bone. 2002;30:178–84.
Oliver WC, Pharr GM. An improved technique for determining hardness and elastic modulus using load and displacement sensing indentation experiments. J Mater Res. 1992;7:1564–83.
Kon E, Muraglia A, Corsi A, Bianco P, Marcacci M, Martin I, Boyde A, Ruspantini I, Chistolini P, Rocca M, Giardino R, Cancedda R, Quarto R. Autologous bone marrow stromal cells loaded onto porous hydroxyapatite ceramic accelerate bone repair in critical-size defects of sheep long bones. J Biomed Mater Res. 2000;49:328–37.
Niemeyer P, Fechner K, Milz S, Richter W, Suedkamp NP, Mehlhorn AT, Pearce S, Kasten P. Comparison of mesenchymal stem cells from bone marrow and adipose tissue for bone regeneration in a critical size defect of the sheep tibia and the influence of platelet-rich plasma. Biomaterials. 2010;31:3572–9.
da Silva Meirelles L, Fontes AM, Covas DT, Caplan AI. Mechanisms involved in the therapeutic properties of mesenchymal stem cells. Cytokine Growth Factor Rev. 2009;20:419–27.
Caplan AI. What’s in a name? Tissue Eng. 2010;16:2415–7.
Hao W, Dong J, Jiang M, Wu J, Cui F, Zhou D. Enhanced bone formation in large segmental radial defects by combining adipose-derived stem cells expressing bone morphogenetic protein 2 with nHA/RHLC/PLA scaffold. Int Orthop. 2010;34:1341–9.
Pearce AI, Richards RG, Milz S, Schneider E, Pearce SG. Animal models for implant biomaterial research in bone: a review. Eur Cell Mater. 2007;13:1–10.
Fantner GE, Hassenkam T, Kindt JH, Weaver JC, Birkedal H, Pechenik L, Cutroni JA, Cidade GA, Stucky GD, Morse DE, Hansma PK. Sacrificial bonds and hidden length dissipate energy as mineralized fibrils separate during bone fracture. Nat Mater. 2005;4:612–6.
Mercer C, He MY, Wang R, Evans AG. Mechanisms governing the inelastic deformation of cortical bone and application to trabecular bone. Acta Biomater. 2006;2:59–68.
Pelled G, Tai K, Sheyn D, Zilberman Y, Kumbar S, Nair LS, Laurencin CT, Gazit D, Ortiz C. Structural and nanoindentation studies of stem cell-based tissue-engineered bone. J Biomech. 2007;40:399–411.
Kang JW, Kang KS, Koo HC, Park JR, Choi EW, Park YH. Soluble factors-mediated immunomodulatory effects of canine adipose tissue-derived mesenchymal stem cells. Stem Cells Dev. 2008;17:681–93.
Yoo KH, Jang IK, Lee MW, Kim HE, Yang MS, Eom Y, Lee JE, Kim YJ, Yang SK, Jung HL, Sung KW, Kim CW, Koo HH. Comparison of immunomodulatory properties of mesenchymal stem cells derived from adult human tissues. Cell Immunol. 2009;259:150–6.
Uccelli A, Moretta L, Pistoia V. Mesenchymal stem cells in health and disease. Nat Rev Immunol. 2008;8:726–36.
Constantin G, Marconi S, Rossi B, Angiari S, Calderan L, Anghileri E, Gini B, Bach SD, Martinello M, Bifari F, Galie M, Turano E, Budui S, Sbarbati A, Krampera M, Bonetti B. Adipose-derived mesenchymal stem cells ameliorate chronic experimental autoimmune encephalomyelitis. Stem Cells. 2009;27:2624–35.
Acknowledgments
The authors are sincerely grateful to Dr. A. Addis, Mr. P. Stortini, and Dr. A. Maragno for their precious work, and to Finceramica S.p.A. (Faenza, Italy) for the appropriately designed scaffolds. This work was partially supported by the Italian Ministry of Health (2007-656853) and a university grant (FIRST-2008).
Author information
Authors and Affiliations
Corresponding author
Additional information
E. Arrigoni and L. de Girolamo contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
About this article
Cite this article
Arrigoni, E., de Girolamo, L., Di Giancamillo, A. et al. Adipose-derived stem cells and rabbit bone regeneration: histomorphometric, immunohistochemical and mechanical characterization. J Orthop Sci 18, 331–339 (2013). https://doi.org/10.1007/s00776-012-0349-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00776-012-0349-y