Abstract
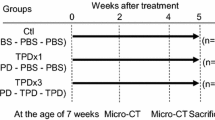
Osteopenia and osteoporosis predominately occur in the fully grown skeleton. However, it is unknown whether disuse osteopenia in skeletally mature, but growing, mice resembles that of fully grown mice. Twenty-four 16-week-old (young) and eighteen 44-week-old (aged) female C57BL/6J mice were investigated. Twelve young and nine aged mice were injected with botulinum toxin in one hind limb; the remaining mice served as controls. The mice were euthanized after 3 weeks of disuse. The femora were scanned by micro-computed tomography (µCT) and bone strength was determined by mechanically testing the femoral mid-diaphysis and neck. At the distal femoral metaphysis, the loss of trabecular bone volume fraction (BV/TV) differed between the young and aged mice. However, at the distal femoral epiphysis, no age-dependent differences were observed. Thinning of the trabeculae was not affected by the age of the mice at either the distal femoral metaphysis or the epiphysis. Furthermore, the aged mice lost more bone strength at the femoral mid-diaphysis, but not at the femoral neck, compared to the young mice. In general, the bone loss induced by botulinum toxin did not differ substantially between young and aged mice. Therefore, the loss of bone in young mice resembles that of aged mice, even though they are not fully grown.






Similar content being viewed by others
References
Shanks N, Greek R, Greek J (2009) Are animal models predictive for humans? Philos Ethics Humanit Med 4:2
Krølner B, Toft B (1983) Vertebral bone loss: an unheeded side effect of therapeutic bed rest. Clin Sci (Lond) 64:537–540
Kovacs CS, Kronenberg HM (1997) Maternal-fetal calcium and bone metabolism during pregnancy, puerperium, and lactation. Endocr Rev 18:832–872
Ebbesen EN, Thomsen JS, Beck-Nielsen H, Nepper-Rasmussen HJ, Mosekilde L (1999) Age- and gender-related differences in vertebral bone mass, density, and strength. J Bone Miner Res 14:1394–1403
Warner SE, Sanford DA, Becker BA, Bain SD, Srinivasan S, Gross TS (2006) Botox-induced muscle paralysis rapidly degrades bone. Bone 38:257–264
Grimston SK, Silva MJ, Civitelli R (2007) Bone loss after temporarily induced muscle paralysis by botox is not fully recovered after 12 weeks. Ann N Y Acad Sci 1116:444–460
Manske SL, Boyd SK, Zernicke RF (2010) Muscle and bone follow similar temporal patterns of recovery from muscle-induced disuse due to botulinum toxin injection. Bone 46:24–31
Lodberg A, Vegger JB, Jensen MV, Larsen CM, Thomsen JS, Brüel A (2015) Immobilization induced bone loss is strain specific in mice. Bone Rep 2:59–67
Vegger JB, Brüel A, Dahlgaard AF, Thomsen JS (2016) Alterations in gene expression precede sarcopenia and osteopenia in botulinum toxin immobilized mice. J Musculoskelet Neuronal Interact 16:355–368
Gall GAE, Kyle WH (1968) Growth of the laboratory mouse. Theor Appl Genet 38:304–308
Rantakokko J, Uusitalo H, Jämsä T, Tuukkanen J, Aro HT, Vuorio E (1999) Expression profiles of mRNAs for osteoblast and osteoclast proteins as indicators of bone loss in mouse immobilization osteopenia model. J Bone Miner Res 14:1934–1942
Jämsä T, Koivukangas A, Ryhänen J, Jalovaara P, Tuukkanen J (1999) Femoral neck is a sensitive indicator of bone loss in immobilized hind limb of mouse. J Bone Miner Res 14:1708–1713
Sakai A, Nakamura T, Tsurukami H, Okazaki R, Nishida S, Tanaka Y, Norimura T, Suzuki K (1996) Bone marrow capacity for bone cells and trabecular bone turnover in immobilized tibia after sciatic neurectomy in mice. Bone 18:479–486
Simske SJ, Greenberg AR, Luttges MW (1991) Effects of suspension-induced osteopenia on the mechanical behaviour of mouse long bones. J Mater Sci Mater Med 2:43–50
Buie HR, Moore CP, Boyd SK (2008) Postpubertal architectural developmental patterns differ between the L3 vertebra and proximal tibia in three inbred strains of mice. J Bone Miner Res 23:2048–2059
Willinghamm MD, Brodt MD, Lee KL, Stephens AL, Ye J, Silva MJ (2010) Age-related changes in bone structure and strength in female and male BALB/c mice. Calcif Tissue Int 86:470–483
Glatt V, Canalis E, Stadmeyer L, Bouxsein ML (2007) Age-related changes in trabecular architecture differ in female and male C57BL/6J mice. J Bone Miner Res 22:1197–1207
Beamer WG, Donahue LR, Rosen CJ, Baylink DJ (1996) Genetic variability in adult bone density among inbred strains of mice. Bone 18:397–403
Bouxsein ML, Boyd SK, Christiansen BA, Guldberg RE, Jepsen KJ, Müller R (2010) Guidelines for assessment of bone microstructure in rodents using micro-computed tomography. J Bone Miner Res 25:1468–1486
Galea GL, Hannuna S, Meakin LB, Delisser PJ, Lanyon LE, Price JS (2015) Quantification of alterations in cortical bone geometry using site specificity software in mouse models of aging and the responses to ovariectomy and altered loading. Front Endocrinol (Lausanne) 6:52
Jilka RL (2013) The relevance of mouse models for investigating age-related bone loss in humans. J Gerontol Ser A Biol Sci Med Sci 68:1209–1217
Donnelly E (2011) Methods for assessing bone quality: a review. Clin Orthop Relat Res 469:2128–2138
Kodama Y, Dimai H, Wergedal J, Sheng M, Malpe R, Kutilek S, Beamer W, Donahue LR, Rosen C, Baylink DJ, Farley J (1999) Cortical tibial bone volume in two strains of mice: effects of sciatic neurectomy and genetic regulation of bone response to mechanical loading. Bone 25:183–190
Lescale C, Schenten V, Djeghloul D, Bennabi M, Gaignier F, Vandamme K, Strazielle C, Kuzniak I, Petite H, Dosquet C, Frippiat JP, Goodhardt M (2015) Hind limb unloading, a model of spaceflight conditions, leads to decreased B lymphopoiesis similar to aging. FASEB J 29:455–463
Meakin LB, Delisser PJ, Galea GL, Lanyon LE, Price JS (2015) Disuse rescues the age-impaired adaptive response to external loading in mice. Osteoporos Int 26:2703–2708
Ellman R, Grasso DJ, van Vliet M, Brooks DJ, Spatz JM, Conlon C, Bouxsein ML (2014) Combined effects of botulinum toxin injection and hind limb unloading on bone and muscle. Calcif Tissue Int 94:327–337
Vegger JB, Brüel A, Thomsen JS (2015) Vertical trabeculae are thinned more than horizontal trabeculae in skeletal-unloaded rats. Calcif Tissue Int 97:516–526
Poliachik SL, Bain SD, Threet D, Huber P, Gross TS (2010) Transient muscle paralysis disrupts bone homeostasis by rapid degradation of bone morphology. Bone 46:18–23
Acknowledgments
The authors are grateful for the hard work and excellent technical assistance of Jytte Utoft. We thank Visiopharm for the contribution to the newCAST stereology software system and the VELUX Foundation for the donation of the µCT scanner. The study was kindly supported by the Health at Aarhus University, Oda and Hans Svennings Foundation, The Vanføre Foundation, Frimodt-Heineke Foundation, Helga and Peter Kornings Foundation, Læge Søren Segel and hustru Johanne Wiibroe Segels Reseachfoundation, Inge og Per Refshalls Researchfoundation, Direktør Jacob Madsen og hustru Olga Madsens Foundation, and the Novo Nordisk Foundation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors have no conflict of interest.
About this article
Cite this article
Vegger, J.B., Brüel, A., Brent, M.B. et al. Disuse osteopenia induced by botulinum toxin is similar in skeletally mature young and aged female C57BL/6J mice. J Bone Miner Metab 36, 170–179 (2018). https://doi.org/10.1007/s00774-017-0830-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00774-017-0830-y