Abstract
To determine the contribution of infant temperament to the relationship between maternal sleep disturbance and depressive symptoms. Utilizing a repeated measures design, 112 couples recruited from childbirth education classes were assessed in third trimester and postpartum. Instruments included Center for Epidemiologic Studies Depression Scale, General Sleep Disturbance Scale, wrist actigraphy, and an investigator-developed tool to assess infant temperament completed by mothers and fathers. Regardless of infant temperament, mothers who slept < 4 h between midnight and 6 am and mothers who napped < 60 min during the day were at increased risk for depression at three months postpartum. Infant temperament was associated with maternal sleep but was not a significant predictor of depressive symptoms after controlling for other contextual factors. Postpartum clinical visits should include questions about maternal sleep so interventions can be directed toward sufficient sleep to minimize risk of postpartum depression.
Similar content being viewed by others
Introduction
Studies suggest that women are more likely than men to develop a major mood disorder in their lifetime, with an increased risk during the childbearing years (Burt and Stein 2002; Altshuler et al. 2001; Beck et al. 1992). The Diagnostic and Statistical Manual of Mental Disorders 4th Edition (DSM-IV) (American Psychiatric Association 1994) defines postpartum depression (PPD) as a constellation of specific symptoms that occur in the first few weeks postpartum while others define PPD as an onset of a depressive episode two weeks to 12 months after giving birth (O’Hara and Swain 1996; Sichel and Driscoll 2002; The Marcé Society 2006). An estimated 400,000 women suffer from PPD each year in the United States (Beck and Gable 2001). Moreover, between 13-20% of all mothers in Western societies are affected by postpartum depression (Dietz et al. 2007; Dobie and Walker 1992; Monti et al. 2008; O’Hara and Swain 1996). Multiple risk factors have been identified in the development of PPD and include: antenatal depression, lack of social support, child-care stress, adolescent pregnancy, poor relationship satisfaction, difficult infant temperament, low self-esteem, and low socioeconomic status (Beck 1996a, 2001; Logsdon and Usui 2001; Hendrick et al. 1998; Studd and Panay 2004; Beck 1995, 1998; Edhborg et al. 2001; Field et al. 1990; Grace et al. 2003; Murray 1992; Kelly et al. 2001; Lindgren 2001).
The postpartum period is an unpredictable time for new mothers and they may find it especially difficult to adapt to the new routines and responsibilities of taking care of a newborn. The addition of a postpartum mood disorder can make the transition to motherhood even more challenging. Difficult infant temperament has been cited as a considerable stressor for the mother in the postpartum period (Cutrona and Troutman 1986; Mayberry and Affonso 1993). In a meta-analysis of 17 studies, a significant correlation was found between infant temperament and PPD (Beck 1996b).
Further examination of the research to date on infant temperament reveals two distinct perspectives. The first suggests that postpartum mood shapes a mother’s perception of her infant (McGrath et al. 2008; Sugawara et al. 1999) and the second suggests that her infant’s temperament influences maternal mood (Beck 1996b; Cutrona and Troutman 1986). However, neither approach considers the sleep disturbance and deprivation that occurs in the postpartum period, and some research designs make it difficult to assess whether maternal sleep loss shapes her perception of her infant, or temperament of her infant influences how much sleep disturbance a mother experiences. Although poor sleep is a risk factor for PPD, and difficult infant temperament is also cited as a risk for developing PPD, there is little research regarding the influence of infant temperament on mother’s sleep and mental health.
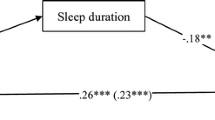
Our previous research has documented the relationship between maternal sleep disturbance and depressive symptoms during the third month postpartum (Goyal et al. 2007). However, the previous study did not consider the role of infant temperament, relationship satisfaction, or other known risk factors for postpartum depression. A framework was developed to help identify contextual factors that may contribute to postpartum depression at three months postpartum. As seen in Fig. 1, salient contextual factors include prenatal depression, mother’s demographic factors (including age, income and education), infant characteristics (health, gender, temperament) and the father of the baby (relationship satisfaction). Therefore, the current study addresses the following two questions in a sample of partnered first-time mothers: 1) is there a relationship between infant temperament and maternal depressive symptoms at 3 months postpartum, and 2) are depressive symptoms associated with sleep disturbance after controlling for other risk factors such as socioeconomic status (age, income, education), history of prenatal depression, relationship satisfaction, and infant characteristics (temperament)?
Materials and methods
Participants
As part of a longitudinal randomized clinical trial to improve parents’ sleep in the first postpartum month, 112 expectant couples were recruited from childbirth education classes during the third trimester of pregnancy. Eligible mothers included nulliparas expecting their first child, partnered, at least 18 years of age, willing to participate, and able to read and write English. Couples were excluded if they planned to hire a live-in nanny, if either parent worked night shift, or if the woman had a history of involuntary pregnancy loss. This study was approved by the institution’s Committee on Human Research. All participants signed the informed consent prior to data collection. All couples were paid for their participation.
Procedure
Women were studied in their homes during their last month of pregnancy and at 1 months, 2 months, and 3 months postpartum. Mothers randomly assigned to the intervention group (n = 75) were given strategies to improve their postpartum sleep, and mothers assigned to the control group (n = 77) were given comparable attention from the research team in the form of information on how to eat a healthy diet. The intervention was designed to improve sleep during the first 4 weeks of postpartum recovery and there were no group differences on the depression measures or sleep measures at baseline or 3 months postpartum. This study reports on the 112 women with complete data regardless of group assignment.
Data analysis
Descriptive statistics were used to describe sample characteristics, Pearson correlations were used to quantify linear associations between continuous variables, and independent t-tests were used to identify group differences on continuous outcomes. A paired t-test was used to compare maternal and paternal ratings of their infant’s temperament. Hierarchical linear regression analysis was used to determine the relationship between infant temperament reported by the mother and by the father and maternal depressive symptoms (continuous CES-D score) at 3 months postpartum, controlling for prenatal depressive symptoms and contextual factors such as maternal demographics and relationship satisfaction. Regression analysis was also used to determine whether the previously documented relationship between sleep and maternal depressive symptoms persists even after controlling for infant temperament. To refine the measurement of sleep, the present study included both objective actigraphy variables in addition to the subjective sleep disturbance scores included in the previous study. Analyses were conducted using SPSS 14.0 (SPSS, Inc, Chicago) and all tests used a significance level of 0.05.
Measures
Participants were asked to provide information regarding age, race, ethnicity, education, and household income. Postpartum information included type of delivery (cesarean or vaginal), infant sex, type of infant feeding, and maternal work status.
Depressive Symptoms
The 20-Item Center for Epidemiological Studies Depression Scale (CES-D) is widely used to assess the frequency of depressive symptoms in women before and after childbirth (Callahan et al. 1991; Radloff 1977). The instructions ask respondents to think back to feelings and attitudes over the past week and check the response that best describes how often they felt or behaved this way. Responses range from 0 (rarely/none or < 1 day) to 3 (most/all the time or 5–7 days) and total scores range between 0 and 60, with a higher score representing more frequent depressive symptoms. A score ≥ 16 is suggested as a risk factor for depressive illness and need for clinical evaluation. This cutoff score was used in the current study to distinguish mothers with an elevated level of depressive symptomatology (Yonkers and Samson 2000). The CES-D was administered at each assessment. The CES-D has been found to have adequate sensitivity to detect major depression and good internal consistency and test-retest reliability in postpartum first-time mothers (Beeghly et al. 2003). In the current sample, the Cronbach alpha coefficient was .77 in the third trimester and .91 at 3 months postpartum.
Subjective Sleep Disturbance
The General Sleep Disturbance Scale (GSDS) is a 21-item self-report measure of sleep disturbance (Lee 1992). Participants are asked to rate the frequency of specific sleep problems during the past week from 0 (not at all) to 7 (every day). The subscales of the GSDS address problems such as difficulty falling asleep, mid-sleep awakening, early awakening, sleep quality, sleep quantity, daytime sleepiness, and use of medication to promote sleep. The total score ranges between 0 and 147. A higher score is indicative of more frequent sleep disturbance. The GSDS has demonstrated good internal consistency, with a Cronbach alpha coefficient of .88 in samples of employed women and childbearing women (Lee 1992; Lee and DeJoseph 1992). In the current sample, the Cronbach alpha coefficient for the GSDS total score was .81 at 3 months postpartum. The GSDS was administered at each assessment, and this study reports the maternal data obtained at three months postpartum when sleep patterns became more stable.
Objective Sleep Disturbance
In order to obtain objective measures of sleep, participants wore a wrist actigraph (Ambulatory Monitoring Inc., Ardsley, NY) for 48 h at each assessment. Wrist actigraphy provides continuous motion data using a wrist-watch size, battery operated microprocessor that senses motion with a piezo-electric linear accelerometer. Actigraph data were analyzed using Action4 software (Ambulatory Monitoring Inc., Ardsley, NY) and yielded three sleep-related outcome variables: 1) total sleep time (TST) at night as a measure of night-time sleep quantity, 2) time spent awake (WAKE) between midnight and 6:00 am as a measure of night-time sleep disruption, and 3) total daytime sleep (DAY) as a measure of daytime sleep quantity that may mitigate the effects of poor night-time sleep quality and quantity. Wake after sleep onset (WASO) is the variable typically used to measure night-time sleep disruption, but due to the multiple bed times and wake times typical in the postpartum period, the WAKE variable was considered a more valid and reliable measure. Given the lack of data for standardization of dichotomous cut-points for actigraphy variables, cut points for this study were based on previous research findings (Gay et al. 2004; Gay and Lee 2004) and the three actigraphy sleep variables were dichotomized as follows: 1) TST insufficient (< 6 h per night) and sufficient (≥ 6 h per night) sleep quantity, 2) WAKE severe (≥ 2 h awake) and mild (< 2 h) wake time between midnight and 6:00 a.m., and 3) DAY sleepers (≥ 60 total minutes of daytime sleep) and non-sleepers (< 60 total minutes during the day). While wearing the wrist actigraph, each participant also completed a 48-hour sleep diary to record bed times and wake times. The diary was used to facilitate interpretation of actigraphy data. Wrist actigraphy and sleep diaries were used at each assessment, and more stable data from the third postpartum month was used for this analysis.
Infant Temperament
Maternal and paternal perceptions of infant temperament were assessed at 1 months, 2 months, and 3 months postpartum using a brief 12-item investigator-developed questionnaire derived from many other child temperament instruments, particularly the 86-item Early Infant Temperament Questionnaire (Medoff-Cooper 1995; Medoff-Cooper et al. 1993) developed to measure many aspects of temperament. Items selected for this investigator-developed instrument were specific adjectives salient to early newborn moods and behaviors that have potential for impacting parental sleep and included quiet, cuddly, easy to soothe, happy, good, fussy, restless, temperamental, unpredictable, easily upset, awake all the time, and asleep all the time. Mothers and fathers were asked to think back over the past seven days and individually rate these behaviors on a scale of 0 (not at all) to 10 (very). Scores are reported as the mean of the 12 items, and a higher score is indicative of a more difficult infant. Additional space was provided for the parent to describe any additional behaviors. The intra-class correlation for the three monthly evaluations was .826 for the mothers and .705 for the fathers. Good internal consistency reliability was also demonstrated, with Cronbach alpha coefficients of .88 at 1 month, .84 at 2 months, and .77 at 3 months postpartum for mothers. Paternal Cronbach alphas ranged between .80–.84. This paper reports on maternal and paternal ratings of infant temperament at 3 months postpartum.
The 13-item Relationship Satisfaction Scale (RSAT) was used to measure current satisfaction with various aspects of the relationship on a 7-point scale from not at all satisfied (0) to very satisfied (6) (Burns et al. 1994). The RSAT was administered at each assessment, and this study reports the maternal data obtained at three months postpartum. The Cronbach alpha coefficient was .93 at 3 months postpartum.
Results
Sample characteristics
Sample characteristics and descriptive data are presented in Table 1. Prenatal data were collected 3.1 ± 1.5 weeks before delivery and postpartum data collection occurred at 11.3 ± 1.0 weeks after delivery. Zero-order correlations among contextual variables, infant temperament, maternal sleep, and maternal depressive symptoms are presented in Table 2. Given the small number of Asian, Hispanic, and African-American participants, as well as participants of mixed or other racial identity, these groups were collapsed for comparison with the Caucasian participants in this sample. There were no racial differences with respect to postpartum depressive symptoms, infant temperament ratings, or maternal perceptions of sleep disturbance, although actigraphy data indicated that non-Caucasian women obtained less sleep at night (6.4 ± 1.1 h) than Caucasian women (7.0 ± 1.2 h; t[110] = 2.45, p = .016). Delivery type, infant’s sex, and feeding type were unrelated to depressive symptoms, infant temperament ratings, or maternal perceptions of postpartum sleep disturbance.
Self-report measures
Maternal and paternal ratings of infant temperament were correlated (r = .40, p < .001), although, on average, fathers rated their infants as more difficult than mothers (paired t[111] = 2.47, p = .015). As shown in Table 2, more difficult infant temperament as rated by both mothers and fathers was weakly associated with maternal subjective report of sleep disturbance. Maternal ratings of infant temperament were weakly associated with mother’s total sleep time at night; although none of the correlations between infant temperament and actigraphy measures were statistically significant. The correlations between parental ratings of infant temperament and maternal depressive symptoms at three months postpartum were in the presumed direction, but did not reach statistical significance. Given the possibility that infant temperament ratings may have been influenced by the extent of parents’ sleep loss, depressed mood, and contact with the infant, regression analyses included both maternal and paternal ratings of infant temperament.
Predicting postpartum depressive symptoms
Hierarchical regression analysis was used to test a model for predicting maternal depressive symptoms at three months postpartum (see Table 3). The overall model explained 54.7% of the variance in postpartum depressive symptoms and the following factors were included:
Socio-demographic Factors
Maternal age, income, education, sex of the infant, delivery type, feeding type, and relationship satisfaction explained 14.5% of the variance in postpartum CES-D scores, with relationship satisfaction being the only significant socio-demographic predictor (β in the final model = −0.15, p = .039).
Prenatal Depressive Symptoms
Given that a history of depressive symptoms is a strong predictor of PPD (Beck 1996a; Logsdon and Usui 2001), third trimester CES-D scores were included in Step 2 of the regression model. Controlling for socio-demographic variables, prenatal depressive symptoms explained 10.0% of the variance in postpartum depression scores (β in the final model = 0.19, p = .012).
Infant Temperament
Controlling for the influence of socio-demographic factors and prenatal depressive symptoms, maternal and paternal ratings of infant temperament explained less than 1% of the variance in maternal depressive symptoms at three months postpartum, and neither parent’s temperament rating made a significant contribution to the model.
Objective Sleep Measures
Controlling for the variables described above, objective actigraphy sleep measures explained 16.4% of the variance in postpartum depressive symptoms. Sleeping at least 1 h during the day was associated with lower postpartum depressive symptom scores (β in the final model = -.18, p = .023) and spending two or more hours awake between 12 AM and 6 AM was associated with higher depressive symptom scores at three months postpartum (β in the final model = 0.37, p < .001).
Subjective Sleep Measures
Even controlling for all of the previous variables, subjective report of sleep disturbance was a significant predictor of postpartum depressive symptoms (β = 0.41, p < .001) and explained an additional 13.4% of the variance in postpartum CES-D scores. Regardless of other factors, including objective measures of sleep quality and quantity, mothers who felt they were not obtaining adequate sleep, either in the GSDS subscale for difficulty falling asleep or subscale for excessive daytime sleepiness, had higher depressive symptom scores in the postpartum period (Table 4).
Discussion and conclusion
The purpose of this study was to describe the relationship between infant temperament and maternal depressive symptoms and to build on our earlier research to identify factors that may change the relationship between sleep and depressive symptoms at 3 months postpartum. The contextual environment framework developed to guide this study identified multiple factors important in the development of PPD (Fig. 1). Socioeconomic status, relationship satisfaction, prenatal depressive symptoms, infant sex, infant temperament, and disrupted maternal sleep have all been implicated in the development of postpartum depressive symptoms (Beck 1996a, 2001; Chaudron et al. 2001; Logsdon and Usui 2001). In our sample of partnered first-time mothers, 28% had depressive symptoms (CES-D ≥ 16) during the third trimester. This high percentage confirms other research where prenatal depression rates as high as 50% have been suggested (Chaudron et al. 2001; Chaudron et al. 2004). In our findings, the risk factors listed above collectively accounted for just over half of the variance in depressive symptoms at three months postpartum (Fig. 1).
Sociodemographic factors such as maternal age, income, education, sex of the infant, delivery type, feeding type, and relationship satisfaction explained 14.5% of the variance in depression symptoms at 3 months postpartum, with only maternal perception of relationship satisfaction being a significant predictor. Our results support a meta-analysis that concluded poor prenatal marital satisfaction was a significant predictor of postpartum depression (Beck 2002).
No correlation was found between infant temperament and maternal depressive symptoms at 3 months postpartum. This was an unexpected finding given the considerable literature suggesting an association between PPD and difficult infant temperament at various infant ages (Austin et al. 2005; Beck 2001; Boyd et al. 2006; Cutrona and Troutman 1986; Edhborg et al. 2005; Galler et al. 2004; Mayberry and Affonso 1993; McGrath et al. 2008; McMahon et al. 2001; O’Hara and Swain 1996; Pauli-Pott et al. 2005; Sugawara et al. 1999). Multiple study designs and methodological differences in how temperament is assessed and by whom make it difficult to compare these varying results. Cutrona and Troutman (1986) were among the first researchers to suggest a relationship between infant difficulty and PPD. They noted that infant temperament assessed by observation and maternal report accounted for 30% of the variance in depressive symptoms using the BDI at three months postpartum. However, their sample of 55 women was primarily married and college educated, and other risk factors such as prenatal depression were not addressed. Cutrona and Troutman’s sample was very similar in age and socioeconomic status to our sample, however, their inclusion of both multiparous women and first-time mothers make comparisons difficult.
Although McGrath and colleagues (2008) found that depressed mothers were more likely to report increased infant difficulty at 2 and 6 months postpartum compared to non-depressed mothers, infant temperament was assessed with a 3-item measure that may have not been adequate to fully assess maternal perception of this construct. Sugawara and colleagues (1999) suggested that maternal depressive symptoms at 5 days postpartum significantly correlated with maternal perception of infant temperament at 6 months postpartum. However, maternal depressive symptoms and infant temperament were assessed at different time intervals from each other and the interval ranged from 5 days to 18 months postpartum. Findings were supported in a longitudinal study of 226 healthy women from Barbados (Galler et al. 2004). Maternal depressive symptoms were measured at 7 weeks and 6 months postpartum, and maternal perception of infant temperament was measured at 7 weeks, 3 and 6 months postpartum. Mothers who reported depressive symptoms at 6 months postpartum were more likely to rate their infant as more difficult than women who were not depressed. Clearly there is a growing body of research suggesting a relationship between infant temperament and PPD. However, it is important to acknowledge that our short investigator-developed measure was internally consistent and stable across the 3 monthly assessments by both mothers and fathers, but has not yet been validated against other more established measures of early infant temperament. The difficulty in comparing results, given differences in prior research designs, methodologies, measurements, and time intervals for these studies should be acknowledged.
Prior research suggests that mothers with PPD are more likely to give negative descriptions of their children than control mothers (Murray and Cooper 1997). Moreover, infant temperament is most often measured by maternal self-report ratings which may be influenced by both depressive symptoms and sleep disturbance. To account for any maternal report bias, regression analyses in this study included both maternal and paternal ratings of infant temperament. Furthermore, both objective and subjective measures of sleep were used in the analyses. Regression analysis identified wake time of more than 2 h between midnight and 6:00 AM, daytime sleep of less than 1 hour, and higher subjective sleep disturbance with more daytime sleepiness (reflected by self-report GSDS score) as significant predictors of maternal depressive symptoms at three months postpartum, after controlling for prenatal depressive symptoms, infant temperament, and relationship satisfaction with the father of the baby.
The GSDS subscale addressing problems of waking up during the night was not correlated with postpartum depressive symptoms. Due to the demands of providing infant care, however, most women had high scores on this scale regardless of their affective state. Our findings are similar to Huang and colleagues (2004) who noted that Taiwanese primiparas with depressive symptoms were more likely report increased daytime sleepiness related to infant care when assessed between 13–20 days postpartum. In our sample, the GSDS subscale addressing excessive daytime sleepiness was significantly correlated with postpartum depressive symptoms. The daytime sleepiness may be due in part to infant care during the night or to the inability or lack of opportunity to nap during the day.
The GSDS subscale for perception of difficulty falling asleep was also highly correlated with postpartum depressive symptoms. In most adults who experience daytime sleepiness, there is no problem with falling asleep at night, and in most adults with initiation insomnia, difficulty falling asleep at night is often associated with higher levels of arousal and hence daytime alertness. This initiation insomnia component of sleep disturbance needs further investigation in samples of women clinically diagnosed with PPD.
Research limitations and implications
This research study was limited by several factors, including the convenience sample design and high socioeconomic status of participants. The rate of clinically at-risk women in our sample (16.4%) was, however, similar to other rates reported in the literature and similar to what would be expected in the general population. To decrease family burden during data collection, infant temperament was assessed with an investigator-developed brief instrument adapted from more extensive measures and focused on early infant behaviors most likely to disrupt parental sleep. All of the participants were partnered and the sample was older than the national average age for first-time mothers in the United States. Over 75% of the sample self-identified as Caucasian, and most had access to private health insurance, indicating greater socioeconomic advantage. Further research in a sample of low-income, younger, culturally diverse women is indicated. Nevertheless, infant temperament assessed by either mother or father did not alter the relationship between poor maternal sleep and depressive symptoms. Further work needs to be done to validate this short infant temperament scale with other more established questionnaires.
In order to further our understanding of why some women are at a higher risk for developing postpartum depression, a contextual environment framework was developed from the study findings that can be tested in future research. Suggestions for future research include using alternative measures for infant temperament, addition of objective infant sleep measures with ankle actigraphy, and longitudinal data collection that follows women later into their first postpartum year.
In conclusion, there is a growing body of literature suggesting a strong relationship between difficult infant temperament and subsequent maternal depressive symptoms. However, many studies have not taken into account other potential predictors of PPD that include parity, partner status and relationship satisfaction, ante-partum depression, and impaired maternal sleep. Our findings suggested maternal and paternal infant temperament ratings are stable over the first three months of life, are moderately correlated, and account for less than 1% of the variance in postpartum depressive symptoms when other factors are considered. Moreover, regardless of infant temperament, new mothers who slept < 4 h between midnight and 6 am, or new mothers who napped < 60 min during the day, were at an increased risk for PPD. Mothers who specifically complain about difficulty falling asleep at bedtime should also be evaluated more extensively for PPD. Healthcare providers should include sleep assessments during postpartum visits and interventions should be directed toward how mothers can obtain enough sleep so that daytime sleepiness is minimized and maternal-infant interactions are promoted to assure healthy infant development.
References
Altshuler, L. L., Cohen, L. S., Moline, M. L., Kahn, D. A., Carpenter, D., & Docherty, J. P. (2001). The expert consensus guideline series. Treatment of depression in women. Postgraduate Medicine (Spec No), 1–107.
American Psychiatric Association (1994) Diagnostic and statistical manual of mental disorders: DSM-IV, 4th edn. American Psychiatric Association, Washington
Austin MP, Hadzi-Pavlovic D, Saint K, Parker G (2005) Antenatal screening for the prediction of postnatal depression: validation of a psychosocial pregnancy risk questionnaire. Acta Psychiatr Scand 112:310–317
Beck CT (1995) The effects of postpartum depression on maternal-infant interaction: a meta-analysis. Nurs Res 44:298–304
Beck CT (1996a) A meta-analysis of predictors of postpartum depression. Nurs Res 45:297–303
Beck CT (1996b) A meta-analysis of the relationship between postpartum depression and infant temperament. Nurs Res 45:225–230
Beck CT (1998) The effects of postpartum depression on child development: a meta-analysis. Arch Psychiatr Nurs 12:12–20
Beck CT (2001) Predictors of postpartum depression: an update. Nurs Res 50:275–285
Beck CT (2002) Postpartum depression: a meta-synthesis. Qual Health Res 12:453–472
Beck CT, Gable RK (2001) Further validation of the postpartum depression screening scale. Nurs Res 50:155–164
Beck CT, Reynolds MA, Rutowski P (1992) Maternity blues and postpartum depression. J Obstet Gynecol Neonatal Nurs 21:287–293
Beeghly M, Olson KL, Weinberg MK, Pierre SC, Downey N, Tronick EZ (2003) Prevalence, stability, and socio-demographic correlates of depressive symptoms in black mothers during the first 18 months postpartum. Matern Child Health J 7:157–168
Boyd RC, Zayas LH, McKee MD (2006) Mother-infant interaction, life events and prenatal and postpartum depressive symptoms among urban minority women in primary care. Matern Child Health J 10:139–148
Burns D, Sayers S, Moras K (1994) Intimate relationships and depression: is there a causal connection? J Consult Clin Psychol 62:1033–1043
Burt VK, Stein K (2002) Epidemiology of depression throughout the female life cycle. J Clin Psychiatry 63(Suppl 7):9–15
Callahan LF, Kaplan MR, Pincus T (1991) The Beck depression inventory, center for epidemiological studies depression scale (CES-D), and general well-being schedule depression subscale in rheumatoid arthritis. Criterion contamination of responses. Arthritis Care Res 4:3–11
Chaudron LH, Klein MH, Remington P, Palta M, Allen C, Essex MJ (2001) Predictors, prodromes and incidence of postpartum depression. J Psychosom Obstet Gynaecol 22:103–112
Chaudron LH, Szilagyi PG, Kitzman HJ, Wadkins HI, Conwell Y (2004) Detection of postpartum depressive symptoms by screening at well-child visits. Pediatrics 113(3 Pt 1):551–558
Cutrona CE, Troutman BR (1986) Social support, infant temperament, and parenting self-efficacy: a mediational model of postpartum depression. Child Dev 57:1507–1518
Dietz PM, Williams SB, Callaghan WM, Bachman DJ, Whitlock EP, Hornbrook MC (2007) Clinically identified maternal depression before, during, and after pregnancies ending in live births. Am J Psychiatry 164:1515–1520
Dobie SA, Walker EA (1992) Depression after childbirth. J Am Board Fam Pract 5:303–311
Edhborg M, Lundh W, Seimyr L, Widstrom AM (2001) The long term impact of postnatal depressed mood on mother-child interaction: a preliminary study. J Reprod Infant Psychol 19:61–71
Edhborg M, Matthiesen AS, Lundh W, Widstrom AM (2005) Some early indicators for depressive symptoms and bonding 2 months postpartum-a study of new mothers and fathers. Archives of Women’s Mental Health 8:221–231
Field T, Healy B, Goldstein S, Guthertz M (1990) Behavior-state matching and synchrony in mother infant interactions of non-depressed versus depressed dyads. Dev Psychol 26:7–14
Galler JR, Harrison RH, Ramsey F, Butler S, Forde V (2004) Postpartum maternal mood, feeding practices, and infant temperament in Barbados. Infant Behav Dev 27:267–287
Gay C, Lee K (2004) Sleep in late pregnancy predicts length of labor and type of delivery. Am J Obstet Gynecol 191(6):2041–2046
Gay C, Lee KA, Lee SY (2004) Sleep patterns and fatigue in new mothers and fathers. Biological Research for Nursing 5(4):311–318
Goyal D, Gay CL, Lee KA (2007) Patterns of sleep disruption and depressive symptoms in new mothers. J Perinat Neonatal Nurs 21:123–129
Grace SL, Evindar A, Stewart DE (2003) The effect of postpartum depression on child cognitive development and behavior: a review and critical analysis of the literature. Archives of Women’s Mental Health 6:263–274
Hendrick V, Altshuler LL, Suri R (1998) Hormonal changes in the postpartum and implications for postpartum depression. Psychosomatics 39:93–101
Kelly R, Zatzick D, Anders T (2001) The detection and treatment of psychiatric disorders and substance use among pregnant women cared for in obstetrics. Am J Psychiatry 158:213–219
Lee KA (1992) Self-reported sleep disturbances in employed women. Sleep 15:493–498
Lee KA, DeJoseph JF (1992) Sleep disturbances, vitality, and fatigue among a select group of employed childbearing women. Birth 19:208–213
Lindgren K (2001) Relationships among maternal-fetal attachment, prenatal depression, and health practices in pregnancy. Res Nurs Health 24:203–217
Logsdon MC, Usui W (2001) Psychosocial predictors of postpartum depression in diverse groups of women. West J Nurs Res 23:563–574
Mayberry LJ, Affonso DD (1993) Infant temperament and postpartum depression: a review. Health Care Women Int 14:201–211
McGrath JM, Records K, Rice M (2008) Maternal depression and infant temperament characteristics. Infant Behav Dev 31:71–80
McMahon C, Barnett B, Kowalenko N, Tennant C, Don N (2001) Postnatal depression, anxiety and unsettled infant behaviour. Aust NZ J Psychiatry 35:581–588
Medoff-Cooper B (1995) Infant temperament: implications for parenting from birth through 1 year. J Pediatr Nurs 10(3):141–145
Medoff-Cooper B, Carey WB, McDevitt SC (1993) The early infancy temperament questionnaire. J Dev Behav Pediatr 14(4):230–235
Monti F, Agostini F, Marano G, Lupi F (2008) The course of maternal depressive symptomatology during the first 18 months postpartum in an Italian sample. Archives of Women’s Mental Health 11:231–238
Murray L (1992) The impact of postnatal depression on infant development. J Child Psychol Psychiatry 33:543–561
Murray L, Cooper P (1997) Effects of postnatal depression on infant development. Arch Dis Child 77:99–101
O’Hara MW, Swain AM (1996) Rates and risk of postpartum depression: a meta-analysis. Int Rev Psychiatry 8:37–54
Pauli-Pott U, Mertesacker B, Beckmann D (2005) Comparing assessment methods of infant emotionality. Zeitschrift fur Kinder- und Jugendpsychiatrie und Psychotherapie 33:123–135
Radloff L (1977) A self-report depression scale for research in the general population. Journal of Applied Psychological Measurement 1:385–401
Sichel D, Driscoll JW (2002) Care for women with postpartum depression: “N*U*R*S*E” approach. Journal of Midwifery & Women’s Health 47:392
Studd J, Panay N (2004) Hormones and depression in women. Climacteric 7:338–346
Sugawara M, Kitamura T, Toda MA, Shima S (1999) Longitudinal relationship between maternal depression and infant temperament in a Japanese population. J Clin Psychol 55:869–880
The Marcé Society. (2006). Retrieved November 10, 2008, from www.marcesociety.com
Yonkers KA, Samson J (2000) Mood disorders measures. In: Rush AJ (ed) Handbook of psychiatric measures. American Psychiatric Association, Washington, pp 515–548
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Goyal, D., Gay, C. & Lee, K. Fragmented maternal sleep is more strongly correlated with depressive symptoms than infant temperament at three months postpartum. Arch Womens Ment Health 12, 229–237 (2009). https://doi.org/10.1007/s00737-009-0070-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00737-009-0070-9