Abstract
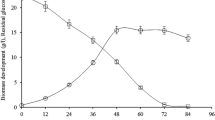
l-DOPA (3,4-dihydroxyphenyl-l-alanine), an amino acid derivative is the most widely used drug of choice for the treatment of Parkinson’s disease and other neurologic injuries. The present study deals with the elevated biochemical transformation of l-tyrosine to l-DOPA by Aspergillus niger PA2, a potent tyrosinase producer, isolated from decomposed food wastes. This appears to be the first report on A. niger as a notable extracellular tyrosinase producer. The extracellular tyrosinase activity produced remarkably higher levels of l-DOPA, i.e. 2.44 mg mL−1 when the media was supplemented with 5 mg mL−1 l-tyrosine. The optimum pH for tyrosinase production was 6.0, with the maximal l-DOPA production at the same pH. The product thus produced was analyzed by thin-layer chromatography, UV spectroscopy, high-performance liquid chromatography and Fourier transform infrared spectroscopy, that had denoted this to be l-DOPA. Kinetic parameters viz. Y p/s, Q s and Q p had further indicated the notable levels of production. Thus, Aspergillus niger PA2 could be a promising resource and may be further exploited for large-scale production of l-DOPA.







Similar content being viewed by others
References
Agarwal P, Saroj S, Dubey S, Singh RP (2014) l-Tyrosinase— a multifunctional enzyme: structural and molecular features. In: Thakur JK (ed) Gene and Protein Engineering, 5th edn. Studium Press, LLC, Houston, pp 427–444
Algieri C, Donato L, Bonacci P, Giorno L (2012) Tyrosinase immobilized on polyamide tubular membrane for the l-DOPA production: total recycle and continuous reactor study. Biochem Eng J 66:14–19
Ali S, Haq I (2006) Kinetic basis of celite (CM 2:1) addition on the biosynthesis of 3,4-dihydroxyphenyl-l-alanine (l-DOPA) by Aspergillus oryzae ME2 using l-tyrosine as a basal substrate. World J Microbiol Biotechnol 22:347–353
Ali S, Haq I (2010) Production of 3,4 dihydroxy l-phenylalanine by a newly isolated Aspergillus niger and parameter significance analysis by Plackett Burman design. BMC Biotechnol 10:86
Ali S, Shultz JL, Haq I (2007) High performance microbiological transformation of l-tyrosine to l-dopa by Yarrowia lipolytica NRRL-143. BMC Biotechnol 7:50
Arica MY, Bayramoglu G, Bicak N (2004) Characterization of tyrosinase immobilized onto spacer-arm attached glycidyl methacrylate-based reactive microbeads. Process Biochem 39:2007–2017
Arnow EL (1937) Colorimetric determination of the components of l-3, 4-dihydroxy phenyl alanine–tyrosine mixtures. J Biol Chem 118:531–537
Burton SG (2003) Oxidizing enzymes as biocatalysts. Trends Biotechnol 21:543–549
Chen T, Vazquez-Duhalt R, Wu CF, Bentley WE, Payne GF (2001) Combinatorial screening for enzyme-mediated coupling. tyrosinase-catalyzed coupling to create protein–chitosan conjugates. Biomacromolecules 2:456–462
Claus H, Decker H (2006) Bacterial tyrosinases. Syst Appl Microbiol 29:3–14
Claus H, Filip Z (1988) Behaviour of phenoloxidases in the presence of clays and other soil-related adsorbents. Appl Microbiol Biotechnol 28:506–511
Gasparetti C, Nordlund E, Jänis J, Buchert J, Kruus K (2012) Extracellular tyrosinase from the fungus Trichoderma reesei shows product inhibition and different inhibition mechanism from the intracellular tyrosinase from Agaricus bisporus. Biochim Biophys Acta 1824:598–607
Gelder CWG, Flurkey WH, Wichers HJ (1997) Sequence and structural features of plant and fungal tyrosinases. Phytochemistry 45:1309–1323
Gunendi G, Pamuk F (1999) Thin layer chromatographic separation and quantitation of l-dopa and l-tyrosine in mixtures. Turk J Chem 23:269–273
Halaouli S, Asther M, Sigoillot JC, Hamdi M, Lomascolo A (2006) Fungal tyrosinases: new prospects in molecular characteristics, bioengineering and biotechnological applications. J Appl Microbiol 100:219–232
Haneda K, Watanabe S, Takeda I (1971) Synthesis of l-3,4-dihydroxyphenylalanine from l-tyrosine by microorganisms. Appl Microbiol 22:721–722
Haq I, Ali S, Qadeer MA, Iqbal J (2003) Inducive effect of cresoquinone on microbiological transformation of l-tyrosine to 3,4 dihydroxy phenyl l-alanine by Aspergillus oryzae NG-11P1. Appl Microbiol Biotechnol 60:696–699
Krishnaveni R, Rathod V, Thakur MS, Neelgund YF (2009) Transformation of l-Tyrosine to l-DOPA by a novel fungus, Acremonium rutilum under submerged fermentation. Curr Microbiol 58:122–128
Kura AU, Hussein SH, Hussein MZ, Fakurazi S, Arulselvan P (2013) Development of a controlled-release anti-parkinsonian nanodelivery system using levodopa as the active agent. Int J Nanomed 8:1103–1110
Lee SG, Rao HS, Hong SP, Kim EH, Sung MH (1996) Production of l-dopa by thermostable tyrosine phenol-lyase of a thermophilic Symbiobacterium spp. over expressed in recombinant Escherichia coli. J Microb Biotechnol 6:98–102
Lerch K (1981) Copper monooxygenases: tyrosinase and dopamine 1-monooxygenase. In: Siegel H (ed) Metal ions in biological systems. Marcel Dekker, New York, pp 143–186
Lewitt PA (2009) Levodopa therapeutics for Parkinson’s disease: new developments. Parkinsonism Relat Disord 15:S31–S34
Liu N, Zhang T, Wang YJ, Huang YP, Ou JH, Shen P (2004) A heat inducible tyrosinase with distinct properties from Bacillus thuringiensis. Lett Appl Microbiol 39:407–412
Mason HS (2003) Mechanism of the oxidation of dihydroxyphenylalanine by tyrosinase. J Biol Chem 172:83–99
Mayer AM, Harel E (1978) Polyphenol oxidases in plants. Phytochemistry 18:193–215
Para GM, Baratti JC (1984) Effect of culture conditions on the production of tyrosine phenol-lyase by Erwinia herbicola. Appl Environ Microbiol 48:1256–1258
Pirt SJ (1975) Principles of cell and microbe cultivation. Blackwell, London
Pollard DJ, Woodley JM (2007) Biocatalysis for pharmaceutical intermediates: the future is now. Trends Biotechnol 25:66–73
Rani N, Joy B, Abraham TE (2007) Cell suspension cultures of Portulaca grandiflora as potent catalysts for biotransformation of l-tyrosine into l-DOPA, an anti-Parkinson’s drug. Pharm Biol 45:48–53
Rao A, Pimprikar P, Bendigiri C, Kumar AR, Zinjarde S (2011) Cloning and expression of a tyrosinase from Aspergillus oryzae in Yarrowia lipolytica: application in l-DOPA biotransformation. Appl Microbiol Biotechnol 92:951–959
Raval KM, Vaswani PS, Majumder DR (2012) Biotransformation of a single amino-acid l-Tyrosine into a bioactive molecule l-DOPA. Int J Sci Res Publ 2(5):2250–3153
Sukumaram CP, Singh DV, Khedkar PD, Mahadevan PR (1979) An actinomycete producing l-3,4-dihydroxyphenylalanine from l-tyrosine. J Biosci 1:236–239
Surwase SN, Jadhav JP (2011) Bioconversion of l-tyrosine to l-DOPA by a novel bacterium Bacillus sp. JPJ. Amino Acids 41:495–506
Surwase SN, Patil SA, Apine OA, Jadhav JP (2012) Efficient microbial conversion of l-tyrosine to l-DOPA by Brevundimonas sp. SGJ. Appl Biochem Biotechnol 167:1015–1028
Vogel HJ (1956) A convenient growth medium for Neurospora (medium N). Microb Genet Bull 13:42–43
Wan X, Chai B, Liao Y, Su Y, Ye T, Shen P, Chen X (2009) Molecular and biochemical characterization of a distinct tyrosinase involved in melanin production from Aeromonas media. Appl Microbiol Biotechnol 82:261–269
White TJ, Bruns T, Lee S, Taylor J (1990) Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. PCR protocols: a guide to methods and applications. Academic, New York, pp 315–322
Zou Y, Hu W, Jiang A, Ma K (2014) Partial purification and characterization of a novel extracellular tyrosinase from Auricularia auricula. Appl Biochem Biotechnol 172:1460–1469
Acknowledgments
Research fellowships awarded to PA by University Grant Commission, Govt. of India, to NP by Council of Scientific and Industrial Research, Govt. of India, to SS by Department of Biotechnology Govt. of India and to JS by Ministry of Human Resource Development, Govt. of India are gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Handling Editor: J. G. López.
Rights and permissions
About this article
Cite this article
Agarwal, P., Pareek, N., Dubey, S. et al. Aspergillus niger PA2: a novel strain for extracellular biotransformation of l-tyrosine into l-DOPA. Amino Acids 48, 1253–1262 (2016). https://doi.org/10.1007/s00726-016-2174-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00726-016-2174-7