Abstract
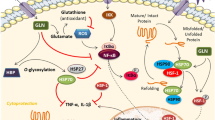
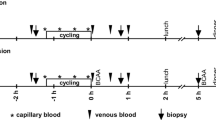
Sustamine™ (SUS) is a dipeptide composed of alanine and glutamine (AlaGln). Glutamine has been suggested to increase muscle protein accretion; however, the underlying molecular mechanisms of glutamine on muscle protein metabolism following resistance exercise have not been fully addressed. In the present study, 2-month-old rats climbed a ladder 10 times with a weight equal to 75 % of their body mass attached at the tail. Rats were then orally administered one of four solutions: placebo (PLA-glycine = 0.52 g/kg), whey protein (WP = 0.4 g/kg), low dose of SUS (LSUS = 0.1 g/kg), or high dose of SUS (HSUS = 0.5 g/kg). An additional group of sedentary (SED) rats was intubated with glycine (0.52 g/kg) at the same time as the ladder-climbing rats. Blood samples were collected immediately after exercise and at either 20 or 40 min after recovery. The flexor hallucis longus (FHL), a muscle used for climbing, was excised at 20 or 40 min post exercise and analyzed for proteins regulating protein synthesis and degradation. All supplements elevated the phosphorylation of FOXO3A above SED at 20 min post exercise, but only the SUS supplements significantly reduced the phosphorylation of AMPK and NF-kB p65. SUS supplements had no effect on mTOR signaling, but WP supplementation yielded a greater phosphorylation of mTOR, p70S6k, and rpS6 compared with PLA at 20 min post exercise. However, by 40 min post exercise, phosphorylation of mTOR and rpS6 in PLA had risen to levels not different than WP. These results suggest that SUS blocks the activation of intracellular signals for MPB, whereas WP accelerates mRNA translation.







Similar content being viewed by others
Abbreviations
- Akt:
-
Protein kinase B
- AMPK:
-
5′ Adenosine monophosphate-activated protein kinase
- FOXO3A:
-
Forkhead box O-3A
- IGF-1:
-
Insulin-like growth factor-1
- mTOR:
-
Mammalian target of rapamycin
- NF-kB p65:
-
Nuclear factor kappa-light-chain-enhancer of activated B cells-p65
- p70S6k:
-
70-kDa ribosomal S6 protein kinase
- rpS6:
-
Ribosomal protein S6
- MPS:
-
Muscle protein synthesis
- MPB:
-
Muscle protein breakdown
References
Anthony JC, Anthony TG, Kimball SR, Jefferson LS (2001) Signaling pathways involved in translational control of protein synthesis in skeletal muscle by leucine. J Nutr 131(3):856S–860S
Anthony TG, McDaniel BJ, Knoll P, Bunpo P, Paul GL, McNurlan MA (2007) Feeding meals containing soy or whey protein after exercise stimulates protein synthesis and translation initiation in the skeletal muscle of male rats. J Nutr 137(2):357–362
Arii K, Kobayashi H, Kai T, Kokuba Y (1999) Degradation kinetics of l-glutamine in aqueous solution. Eur J Pharm Sci 9(1):75–78
Atherton PJ, Etheridge T, Watt PW, Wilkinson D, Selby A, Rankin D et al (2010) Muscle full effect after oral protein: time-dependent concordance and discordance between human muscle protein synthesis and mTORC1 signaling. Am J Clin Nutr 92(5):1080–1088
Attaix D, Ventadour S, Codran A, Bechet D, Taillandier D, Combaret L (2005) The ubiquitin-proteasome system and skeletal muscle wasting. Essays Biochem 41:173–186
Baar K, Esser K (1999) Phosphorylation of p70(S6k) correlates with increased skeletal muscle mass following resistance exercise. Am J Physiol 276(1 Pt 1):C120–C127
Bernard JR, Liao YH, Doerner PG 3rd, Ding Z, Hsieh M, Wang W et al (2012) An amino acid mixture is essential to optimize insulin-stimulated glucose uptake and GLUT4 translocation in perfused rodent hind limb muscle. J Appl Physiol (1985) 113(1):97–104
Bolster DR, Kubica N, Crozier SJ, Williamson DL, Farrell PA, Kimball SR et al (2003) Immediate response of mammalian target of rapamycin (mTOR)-mediated signalling following acute resistance exercise in rat skeletal muscle. J Physiol 553(Pt 1):213–220
Bolster DR, Jefferson LS, Kimball SR (2004) Regulation of protein synthesis associated with skeletal muscle hypertrophy by insulin-, amino acid- and exercise-induced signalling. Proc Nutr Soc 63(2):351–356
Bonetto A, Penna F, Minero VG, Reffo P, Costamagna D, Bonelli G et al (2011) Glutamine prevents myostatin hyper expression and protein hypercatabolism induced in C2C12 myotubes by tumor necrosis factor-alpha. Amino Acids 40(2):585–594
Borsheim E, Tipton KD, Wolf SE, Wolfe RR (2002) Essential amino acids and muscle protein recovery from resistance exercise. Am J Physiol Endocrinol Metab 283(4):E648–E657
Bowtell JL, Gelly K, Jackman ML, Patel A, Simeoni M, Rennie MJ (1999) Effect of oral glutamine on whole body carbohydrate storage during recovery from exhaustive exercise. J Appl Physiol (1985) 86(6):1770–1777
Brunet A, Bonni A, Zigmond MJ, Lin MZ, Juo P, Hu LS et al (1999) Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell 96(6):857–868
Cai D, Frantz JD, Tawa NE Jr, Melendez PA, Oh BC, Lidov HG et al (2004) IKKbeta/NF-kappaB activation causes severe muscle wasting in mice. Cell 119(2):285–298
Cruzat VF, Rogero MM, Tirapegui J (2010) Effects of supplementation with free glutamine and the dipeptide alanyl-glutamine on parameters of muscle damage and inflammation in rats submitted to prolonged exercise. Cell Biochem Funct 28(1):24–30
Felig P (1975) Amino acid metabolism in man. Annu Rev Biochem 44:933–955
Fingar DC, Blenis J (2004) Target of rapamycin (TOR): an integrator of nutrient and growth factor signals and coordinator of cell growth and cell cycle progression. Oncogene 23(18):3151–3171
Fingar DC, Salama S, Tsou C, Harlow E, Blenis J (2002) Mammalian cell size is controlled by mTOR and its downstream targets S6K1 and 4EBP1/eIF4E. Genes Dev 16(12):1472–1487
Fingar DC, Richardson CJ, Tee AR, Cheatham L, Tsou C, Blenis J (2004) mTOR controls cell cycle progression through its cell growth effectors S6K1 and 4E-BP1/eukaryotic translation initiation factor 4E. Mol Cell Biol 24(1):200–216
Fryburg DA, Barrett EJ (1993) Growth hormone acutely stimulates skeletal muscle but not whole-body protein synthesis in humans. Metabolism 42(9):1223–1227
Fryburg DA, Gelfand RA, Barrett EJ (1991) Growth hormone acutely stimulates forearm muscle protein synthesis in normal humans. Am J Physiol 260(3 Pt 1):E499–E504
Furst P (2001) New developments in glutamine delivery. J Nutr 131(9 Suppl):2562S–2568S
Gleeson M (2008) Dosing and efficacy of glutamine supplementation in human exercise and sport training. J Nutr 138(10):2045S–2049S
Goodman CA, Miu MH, Frey JW, Mabrey DM, Lincoln HC, Gen Y et al (2010) A phosphatidylinositol 3-kinase/protein kinase B-independent activation of mammalian target of rapamycin signaling is sufficient to induce skeletal muscle hypertrophy. Mol Biol Cell 21(18):3258–3268
Harris RC, Hoffman JR, Allsopp A, Routledge NB (2012) l-glutamine absorption is enhanced after ingestion of l-alanylglutamine compared with the free amino acid or wheat protein. Nutr Res 32(4):272–277
Hoffman JR, Ratamess NA, Kang J, Rashti SL, Kelly N, Gonzalez AM et al (2010) Examination of the efficacy of acute l-alanyl-l-glutamine ingestion during hydration stress in endurance exercise. J Int Soc Sports Nutr 7:8
Hohorst HJ (1965) Determination of L-lactate with LDH and DPN. In: Bergmeywe HU (ed) Methods of enzymatic analysis. Academic, New York, pp 265–270
Hulmi JJ, Tannerstedt J, Selanne H, Kainulainen H, Kovanen V, Mero AA (2009) Resistance exercise with whey protein ingestion affects mTOR signaling pathway and myostatin in men. J Appl Physiol (1985) 106(5):1720–1729
Jagoe RT, Goldberg AL (2001) What do we really know about the ubiquitin-proteasome pathway in muscle atrophy? Curr Opin Clin Nutr Metab Care 4(3):183–190
Jastrzebski K, Hannan KM, Tchoubrieva EB, Hannan RD, Pearson RB (2007) Coordinate regulation of ribosome biogenesis and function by the ribosomal protein S6 kinase, a key mediator of mTOR function. Growth Factors 25(4):209–226
Jepson MM, Bates PC, Broadbent P, Pell JM, Millward DJ (1988) Relationship between glutamine concentration and protein synthesis in rat skeletal muscle. Am J Physiol 255(2 Pt 1):E166–E172
Lacey JM, Wilmore DW (1990) Is glutamine a conditionally essential amino acid? Nutr Rev 48(8):297–309
Laemmli U (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227(5259):680–685
Lambertucci AC, Lambertucci RH, Hirabara SM, Curi R, Moriscot AS, Alba-Loureiro TC et al (2012) Glutamine supplementation stimulates protein-synthetic and inhibits protein-degradative signaling pathways in skeletal muscle of diabetic rats. PLoS One 7(12):e50390
MacLennan PA, Brown RA, Rennie MJ (1987) A positive relationship between protein synthetic rate and intracellular glutamine concentration in perfused rat skeletal muscle. FEBS Lett 215(1):187–191
Miller SL, Tipton KD, Chinkes DL, Wolf SE, Wolfe RR (2003) Independent and combined effects of amino acids and glucose after resistance exercise. Med Sci Sports Exerc 35(3):449–455
Moore DR, Robinson MJ, Fry JL, Tang JE, Glover EI, Wilkinson SB et al (2009) Ingested protein dose response of muscle and albumin protein synthesis after resistance exercise in young men. Am J Clin Nutr 89(1):161–168
Morrison PJ, Hara D, Ding Z, Ivy JL (2008) Adding protein to a carbohydrate supplement provided after endurance exercise enhances 4E-BP1 and RPS6 signaling in skeletal muscle. J Appl Physiol 104(4):1029–1036
Nakashima K, Yakabe Y (2007) AMPK activation stimulates myofibrillar protein degradation and expression of atrophy-related ubiquitin ligases by increasing FOXO transcription factors in C2C12 myotubes. Biosci Biotechnol Biochem 71(7):1650–1656
Newsholme EA, Parry-Billings M (1990) Properties of glutamine release from muscle and its importance for the immune system. JPEN J Parenter Enteral Nutr 14(4 Suppl):63S–67S
Newsholme P, Procopio J, Lima MM, Pithon-Curi TC, Curi R (2003) Glutamine and glutamate—their central role in cell metabolism and function. Cell Biochem Funct 21(1):1–9
Novak F, Heyland DK, Avenell A, Drover JW, Su X (2002) Glutamine supplementation in serious illness: a systematic review of the evidence. Crit Care Med 30(9):2022–2029
Pende M, Um SH, Mieulet V, Sticker M, Goss VL, Mestan J et al (2004) S6K1(−/−)/S6K2(−/−) mice exhibit perinatal lethality and rapamycin-sensitive 5′-terminal oligopyrimidine mRNA translation and reveal a mitogen-activated protein kinase-dependent S6 kinase pathway. Mol Cell Biol 24(8):3112–3124
Powers SK, Kavazis AN, McClung JM (2007) Oxidative stress and disuse muscle atrophy. J Appl Physiol (1985) 102(6):2389–2397
Rogero MM, Tirapegui J, Pedrosa RG, Pires ISD, de Castro IA (2004) Plasma and tissue glutamine response to acute and chronic supplementation with l-glutamine and l-alanyl-l-glutamine in rats. Nutr Res 24(4):261–270
Rogero MM, Tirapegui J, Pedrosa RG, Castro IA, Pires IS (2006) Effect of alanyl-glutamine supplementation on plasma and tissue glutamine concentrations in rats submitted to exhaustive exercise. Nutrition 22(5):564–571
Roux PP, Blenis J (2004) ERK and p38 MAPK-activated protein kinases: a family of protein kinases with diverse biological functions. Microbiol Mol Biol Rev 68(2):320–344
Salehian B, Mahabadi V, Bilas J, Taylor WE, Ma K (2006) The effect of glutamine on prevention of glucocorticoid-induced skeletal muscle atrophy is associated with myostatin suppression. Metabolism 55(9):1239–1247
Saltiel AR (1996) Diverse signaling pathways in the cellular actions of insulin. Am J Physiol 270(3 Pt 1):E375–E385
Stehle P, Zander J, Mertes N, Albers S, Puchstein C, Lawin P et al (1989) Effect of parenteral glutamine peptide supplements on muscle glutamine loss and nitrogen balance after major surgery. Lancet 1(8632):231–233
Stitt TN, Drujan D, Clarke BA, Panaro F, Timofeyva Y, Kline WO et al (2004) The IGF-1/PI3K/Akt pathway prevents expression of muscle atrophy-induced ubiquitin ligases by inhibiting FOXO transcription factors. Mol Cell 14(3):395–403
Tang JE, Moore DR, Kujbida GW, Tarnopolsky MA, Phillips SM (2009) Ingestion of whey hydrolysate, casein, or soy protein isolate: effects on mixed muscle protein synthesis at rest and following resistance exercise in young men. J Appl Physiol 107(3):987–992
Tipton KD, Ferrando AA, Phillips SM, Doyle D Jr, Wolfe RR (1999) Postexercise net protein synthesis in human muscle from orally administered amino acids. Am J Physiol 276(4 Pt 1):E628–E634
Welbourne TC (1995) Increased plasma bicarbonate and growth hormone after an oral glutamine load. Am J Clin Nutr 61(5):1058–1061
West DW, Kujbida GW, Moore DR, Atherton P, Burd NA, Padzik JP et al (2009) Resistance exercise-induced increases in putative anabolic hormones do not enhance muscle protein synthesis or intracellular signalling in young men. J Physiol 587(Pt 21):5239–5247
Zanchi NE, Filho MA, Felitti V, Nicastro H, Lorenzeti FM, Lancha AH Jr (2010) Glucocorticoids: extensive physiological actions modulated through multiple mechanisms of gene regulation. J Cell Physiol 224(2):311–315
Zhou X, Thompson JR (1997) Regulation of protein turnover by glutamine in heat-shocked skeletal myotubes. Biochim Biophys Acta 1357(2):234–242
Zhu Y, Lin G, Dai Z, Zhou T, Li T, Yuan T et al (2014) l-glutamine deprivation induces autophagy and alters the mTOR and MAPK signaling pathways in porcine intestinal epithelial cells. Amino Acids. doi:10.1007/s00726-014-1785-0
Acknowledgments
We would like to thank Lynne Kammer and all other colleagues in the Exercise Physiology and Metabolism Laboratory at the University of Texas at Austin for the excellent technical assistance.
Conflict of interest
This research was supported by a grant from Kyowa Hakko Bio Co., Ltd.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling Editor: S. L. Parker.
Rights and permissions
About this article
Cite this article
Wang, W., Choi, R.H., Solares, G.J. et al. l-Alanylglutamine inhibits signaling proteins that activate protein degradation, but does not affect proteins that activate protein synthesis after an acute resistance exercise. Amino Acids 47, 1389–1398 (2015). https://doi.org/10.1007/s00726-015-1972-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00726-015-1972-7