Abstract
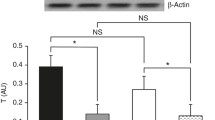
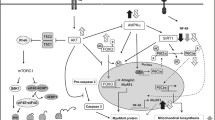
The present study tested the hypothesis that sepsis-induced leucine (Leu) resistance in skeletal muscle is associated with a down-regulation of amino acid transporters important in regulating Leu flux or an impairment in the formation of the Leu-sensitive mTOR–Ragulator complex. Sepsis in adult male rats decreased basal protein synthesis in gastrocnemius, associated with a reduction in mTOR activation as indicated by decreased 4E-BP1 and S6K1 phosphorylation. The ability of oral Leu to increase protein synthesis and mTOR kinase after 1 h was largely prevented in sepsis. Sepsis increased CAT1, LAT2 and SNAT2 mRNA content two- to fourfold, but only the protein content for CAT1 (20 % decrease) differed significantly. Conversely, sepsis decreased the proton-assisted amino acid transporter (PAT)-2 mRNA by 60 %, but without a coordinate change in PAT2 protein. There was no sepsis or Leu effect on the protein content for RagA-D, LAMTOR-1 and -2, raptor, Rheb or mTOR in muscle. The binding of mTOR, PRAS40 and RagC to raptor did not differ for control and septic muscle in the basal condition; however, the Leu-induced decrease in PRAS40·raptor and increase in RagC·raptor seen in control muscle was absent in sepsis. The intracellular Leu concentration was increased in septic muscle, compared to basal control conditions, and oral Leu further increased the intracellular Leu concentration similarly in both control and septic rats. Hence, while alterations in select amino acid transporters are not associated with development of sepsis-induced Leu resistance, the Leu-stimulated binding of raptor with RagC and the recruitment of mTOR/raptor to the endosome-lysosomal compartment may partially explain the inability of Leu to fully activate mTOR and muscle protein synthesis.







Similar content being viewed by others
References
Askanazi J, Carpentier YA, Michelsen CB, Elwyn DH, Furst P, Kantrowitz LR, Gump FE, Kinney JM (1980) Muscle and plasma amino acids following injury. Influence of intercurrent infection. Ann Surg 192:78–85
Avruch J, Long X, Ortiz-Vega S, Rapley J, Papageorgiou A, Dai N (2009) Amino acid regulation of TOR complex 1. Am J Physiol Endocrinol Metab 296:E592–E602
Bar-Peled L, Schweitzer LD, Zoncu R, Sabatini DM (2012) Ragulator is a GEF for the rag GTPases that signal amino acid levels to mTORC1. Cell 150:1196–1208
Blackburn GL, Bistrian BR (1976) Nutritional care of the injured and/or septic patient. Surg Clin North Am 56:1195–1224
Callahan LA, Supinski GS (2009) Sepsis-induced myopathy. Crit Care Med 37:S354–S367
Crozier SJ, Kimball SR, Emmert SW, Anthony JC, Jefferson LS (2005) Oral leucine administration stimulates protein synthesis in rat skeletal muscle. J Nutr 135:376–382
Dennis MD, Baum JI, Kimball SR, Jefferson LS (2011) Mechanisms involved in the coordinate regulation of mTORC1 by insulin and amino acids. J Biol Chem 286:8287–8296
Dodd KM, Tee AR (2012) Leucine and mTORC1: a complex relationship. Am J Physiol Endocrinol Metab 302:E1329–E1342
Drummond MJ, Glynn EL, Fry CS, Timmerman KL, Volpi E, Rasmussen BB (2010) An increase in essential amino acid availability upregulates amino acid transporter expression in human skeletal muscle. Am J Physiol Endocrinol Metab 298:E1011–E1018
Drummond MJ, Dickinson JM, Fry CS, Walker DK, Gundermann DM, Reidy PT, Timmerman KL, Markofski MM, Paddon-Jones D, Rasmussen BB, Volpi E (2012) Bed rest impairs skeletal muscle amino acid transporter expression, mTORC1 signaling, and protein synthesis in response to essential amino acids in older adults. Am J Physiol Endocrinol Metab 302:E1113–E1122
Evans K, Nasim Z, Brown J, Butler H, Kauser S, Varoqui H, Erickson JD, Herbert TP, Bevington A (2007) Acidosis-sensing glutamine pump SNAT2 determines amino acid levels and mammalian target of rapamycin signalling to protein synthesis in L6 muscle cells. J Am Soc Nephrol 18:1426–1436
Fernandez J, Yaman I, Merrick WC, Koromilas A, Wek RC, Sood R, Hensold J, Hatzoglou M (2002) Regulation of internal ribosome entry site-mediated translation by eukaryotic initiation factor-2alpha phosphorylation and translation of a small upstream open reading frame. J Biol Chem 277:2050–2058
Findlay GM, Yan L, Procter J, Mieulet V, Lamb RF (2007) A MAP4 kinase related to Ste20 is a nutrient-sensitive regulator of mTOR signalling. Biochem J 403:13–20
Frost RA, Lang CH (2011) mTor signaling in skeletal muscle during sepsis and inflammation: where does it all go wrong? Physiol (Bethesda) 26:83–96
Goberdhan DC, Ogmundsdottir MH, Kazi S, Reynolds B, Visvalingam SM, Wilson C, Boyd CA (2009) Amino acid sensing and mTOR regulation: inside or out? Biochem Soc Trans 37:248–252
Han JM, Jeong SJ, Park MC, Kim G, Kwon NH, Kim HK, Ha SH, Ryu SH, Kim S (2012) Leucyl-tRNA synthetase is an intracellular leucine sensor for the mTORC1-signaling pathway. Cell 149:410–424
Hatzoglou M, Fernandez J, Yaman I, Closs E (2004) Regulation of cationic amino acid transport: the story of the CAT-1 transporter. Annu Rev Nutr 24:377–399
Heublein S, Kazi S, Ogmundsdottir MH, Attwood EV, Kala S, Boyd CA, Wilson C, Goberdhan DC (2010) Proton-assisted amino-acid transporters are conserved regulators of proliferation and amino-acid-dependent mTORC1 activation. Oncogene 29:4068–4079
Hundal HS, Taylor PM (2009) Amino acid transceptors: gate keepers of nutrient exchange and regulators of nutrient signaling. Am J Physiol Endocrinol Metab 296:E603–E613
Hyde R, Hajduch E, Powell DJ, Taylor PM, Hundal HS (2005) Ceramide down-regulates System A amino acid transport and protein synthesis in rat skeletal muscle cells. FASEB J 19:461–463
Hyde R, Cwiklinski EL, MacAulay K, Taylor PM, Hundal HS (2007) Distinct sensor pathways in the hierarchical control of SNAT2, a putative amino acid transceptor, by amino acid availability. J Biol Chem 282:19788–19798
Jewell JL, Russell RC, Guan KL (2013) Amino acid signalling upstream of mTOR. Nat Rev Mol Cell Biol 14:133–139
Kazi AA, Pruznak AM, Frost RA, Lang CH (2011) Sepsis-induced alterations in protein-protein interactions within mTOR complex 1 and the modulating effect of leucine on muscle protein synthesis. Shock 35:117–125
Kim DH, Sarbassov DD, Ali SM, King JE, Latek RR, Erdjument-Bromage H, Tempst P, Sabatini DM (2002) mTOR interacts with raptor to form a nutrient-sensitive complex that signals to the cell growth machinery. Cell 110:163–175
Lang CH, Frost RA (2004) Differential effect of sepsis on ability of leucine and IGF-I to stimulate muscle translation initiation. Am J Physiol Endocrinol Metab 287:E721–E730
Lang CH, Frost RA (2006) Glucocorticoids and TNFalpha interact cooperatively to mediate sepsis-induced leucine resistance in skeletal muscle. Mol Med 12:291–299
Lang CH, Frost RA (2007) Sepsis-induced suppression of skeletal muscle translation initiation mediated by tumor necrosis factor alpha. Metabolism 56:49–57
Lang CH, Fan J, Cooney R, Vary TC (1996) IL-1 receptor antagonist attenuates sepsis-induced alterations in the IGF system and protein synthesis. Am J Physiol 270:E430–E437
Lang CH, Pruznak AM, Frost RA (2005) TNFalpha mediates sepsis-induced impairment of basal and leucine-stimulated signaling via S6K1 and eIF4E in cardiac muscle. J Cell Biochem 94:419–431
Lang CH, Frost RA, Bronson SK, Lynch CJ, Vary TC (2010) Skeletal muscle protein balance in mTOR heterozygous mice in response to inflammation and leucine. Am J Physiol Endocrinol Metab 298:E1283–E1294
Liu J, Hatzoglou M (1998) Control of expression of the gene for the arginine transporter Cat-1 in rat liver cells by glucocorticoids and insulin. Amino Acids 15:321–337
Mackenzie B, Erickson JD (2004) Sodium-coupled neutral amino acid (System N/A) transporters of the SLC38 gene family. Pflugers Arch 447:784–795
Ogmundsdottir MH, Heublein S, Kazi S, Reynolds B, Visvalingam SM, Shaw MK, Goberdhan DC (2012) Proton-assisted amino acid transporter PAT1 complexes with Rag GTPases and activates TORC1 on late endosomal and lysosomal membranes. PLoS ONE 7:e36616
Roth E, Funovics J, Muhlbacher F, Schemper M, Mauritz W, Sporn P, Fritsch A (1982) Metabolic disorders in severe abdominal sepsis: glutamine deficiency in skeletal muscle. Clin Nutr 1:25–41
Sancak Y, Thoreen CC, Peterson TR, Lindquist RA, Kang SA, Spooner E, Carr SA, Sabatini DM (2007) PRAS40 is an insulin-regulated inhibitor of the mTORC1 protein kinase. Mol Cell 25:903–915
Sancak Y, Bar-Peled L, Zoncu R, Markhard AL, Nada S, Sabatini DM (2010) Ragulator-Rag complex targets mTORC1 to the lysosomal surface and is necessary for its activation by amino acids. Cell 141:290–303
Suryawan A, Nguyen HV, Almonaci RD, Davis TA (2013) Abundance of amino acid transporters involved in mTORC1 activation in skeletal muscle of neonatal pigs is developmentally regulated. Amino Acids 45:523–530
Vary TC, Lang CH (2008) Assessing effects of alcohol consumption on protein synthesis in striated muscles. Methods Mol Biol 447:343–355
Verrey F (2003) System L: heteromeric exchangers of large, neutral amino acids involved in directional transport. Pflugers Arch 445:529–533
Wolfe RR, Jahoor F, Hartl WH (1989) Protein and amino acid metabolism after injury. Diabetes Metab Rev 5:149–164
Zoncu R, Bar-Peled L, Efeyan A, Wang S, Sancak Y, Sabatini DM (2011) mTORC1 senses lysosomal amino acids through an inside-out mechanism that requires the vacuolar H(+)-ATPase. Science 334:678–683
Acknowledgments
This work was supported by National Institutes of Health grant GM38032.
Conflict of interest
The authors state that they have no conflict of interest to declare.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Laufenberg, L.J., Pruznak, A.M., Navaratnarajah, M. et al. Sepsis-induced changes in amino acid transporters and leucine signaling via mTOR in skeletal muscle. Amino Acids 46, 2787–2798 (2014). https://doi.org/10.1007/s00726-014-1836-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00726-014-1836-6