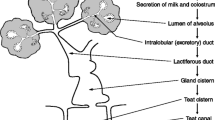
Abstract
Amino acids (AA) are not only building blocks of protein but are also key regulators of metabolic pathways in animals. Understanding the fate of AA is crucial to optimize utilization of AA for milk protein synthesis and, therefore, to reduce inefficiencies of nutrient utilization during lactation. By understanding the functional role of AA metabolism in mammary tissue, we can uncover pathways and molecular targets to improve AA utilization by mothers and neonates during the lactation period. The major objective of this article is to highlight recent advances in mammary AA transport, metabolism and utilization. Such knowledge will aid in refining dietary requirements of AA for lactating mammals, including women, sows and cows.


Similar content being viewed by others
Abbreviations
- AA:
-
Amino acids
- ASCT:
-
Alanine, serine, cysteine amino acid transport protein
- ATB0,+ :
-
B0,+-type amino acid transport protein
- b0,+AT:
-
b0,+-type amino acid transport protein
- BCAA:
-
Branched-chain amino acids
- BCAT:
-
Branched-chain aminotransferase
- BCKA:
-
Branched-chain α-keto acids
- BCKAD:
-
Branched-chain α-keto acid dehydrogenase
- CAT-1:
-
Cationic amino acid transport protein 1
- CAT-2b:
-
Cationic amino acid transport protein 2
- CP:
-
Crude protein
- DETA-NO:
-
Diethylenetriamine-NO
- EAAT:
-
Excitatory amino acid transporter
- GOT:
-
Glutamate oxaloacetate transaminase
- GPT:
-
Glutamate pyruvate transaminase
- GS:
-
Glutamine synthetase
- LAT:
-
L-type amino acid transporter
- NOS:
-
Nitric oxide synthase
- NRC:
-
National Research Council
- OAT:
-
Ornithine aminotransferase
- ODC:
-
Ornithine decarboxylase
- P5C:
-
Δ1-l-Pyrroline-5-carboxylate
- P5CD:
-
Δ1-l-Pyrroline-5-carboxylate dehydrogenase
- P5CR:
-
Δ1-l-Pyrroline-5-carboxylate reductase
- y+LAT:
-
y+L-Type amino acid transporter
- rBAT:
-
Amino acid transporter rB
- SNAT:
-
Sodium-coupled neutral amino acid transporter
- TAT:
-
T-type amino acid transporter
References
Aleman G, Lopez A, Ordaz G, Torres N, Tovar AR (2009) Changes in messenger RNA abundance of amino acid transporters in rat mammary gland during pregnancy, lactation, and weaning. Metabolism 58:594–601
Appuhamy JA, Bell AL, Nayananjalie WA, Escobar J, Hanigan MD (2011) Essential amino acids regulate both initiation and elongation of mRNA translation independent of insulin in MAC-T cells and bovine mammary tissue slices. J Nutr 141:1209–1215
Appuhamy JA, Knoebel NA, Nayananjalie WA et al (2012) Isoleucine and leucine independently regulate mTOR signaling and protein synthesis in MAC-T cells and bovine mammary tissue slices. J Nutr 142:484–491
Arriza J, Kavanaugh M, Fairman W, Wu Y, Murdoch G, North R et al (1993) Cloning and expression of a human neutral amino acid transporter with structural similarity to the glutamate transporter gene family. J Biol Chem 268:15329–15332
Barker GA, Ellory JC (1990) The identification of neutral amino acid transport systems. Exp Physiol 75:3–26
Basch JJ, Wickham ED, Farrell HM, Keys JE (1995) Ornithine-d-aminotransferase in lactating bovine mammary glands. J Dairy Sci 78:825–828
Basch JJ, Wickham ED, Farrell HM (1996) Pyrroline-5-carboxylate reductase in lactating bovine mammary glands. J Dairy Sci 79:1361–1364
Basch JJ, Wickham ED, Farrell HM (1997) Arginase in lactating bovine mammary glands: implications in proline synthesis. J Dairy Sci 80(12):3241–3248
Bauch C, Forster N, Loffing-Cueni D, Summa V, Verrey F (2003) Functional cooperation of epithelial heteromeric amino acid transporters expressed in madin-darby canine kidney cells. Biochem J 278:1316–1322
Baumrucker CR (1985) Amino acid transport systems in bovine mammary tissue. J Dairy Sci 68:2436–2451
Bequette BJ, Douglass LW (2010) The frequency of unilateral milking alters leucine metabolism and amino acid removal by the mammary gland of lactating goats. J Dairy Sci 93:162–169
Bequette BJ, Backwell FRC, MacRae JC, Lobley GE, Crompton LA, Metcalf JA, Sutton JD (1996a) Effect of intravenous amino acid infusion on leucine oxidation across the mammary gland of the lactating goat. J Dairy Sci 79:2217–2222
Bequette BJ, Metcalf JA, Wray-Cahen D, Backwell FR, Sutton JD, Lomax MA, MacRae JC, Lobley GE (1996b) Leucine and protein metabolism in the lactating dairy cow mammary gland: responses to supplemental dietary crude protein intake. J Dairy Res 63:209–222
Bequette BJ, Backwell FRC, Crompton LA (1998) Current concepts of amino acid and protein metabolism by the lactating ruminant mammary gland. J Dairy Sci 8:2540–2559
Bequette BJ, Backwell FRC, Kyle CE, Calder AG, Buchan V, Crompton LA, France J, MacRae JC (1999) Vascular sources of phenylalanine, tyrosine, lysine and methionine for casein synthesis in lactating goats. J Dairy Sci 82:362–377
Bequette BJ, Kyle CE, Crompton LA, Calder AG, Hanigan MD (2002) Protein metabolism in lactating goats subjected to the insulin clamp. J Dairy Sci 85:1546–1555
Bertolo RFP, Brunton JA, Pencharz PB, Ball RO (2003) Arginine, ornithine, and proline interconversion is dependent on small intestinal metabolism in neonatal pigs. Am J Physiol Endocrinol Metab 284:E915–E922
Bionaz M, Loor JJ (2011) Gene networks driving bovine mammary protein synthesis during the lactation cycle. Bioinform Biol Insights 5:83–98
Bröer S (2008) Amino acid transport across mammalian intestinal and renal epithelia. Physiol Rev 88:249–286
Bröer A, Wagner CA, Lang F, Broer S (2000a) The heterodimeric amino acid transporter 4F2hc/y+LAT2 mediates arginine efflux in exchange with glutamine. Biochem J 349:787–795
Bröer A, Wagner CA, Lang F, Broer S (2000b) Neutral amino acid transporter ASCT2 displays substrate-induced Na+ exchange and a substrate-gated anion conductance. Biochem J 346:705–710
Bröer A, Klingel K, Kowalczuk S, Rasko JEJ, Cavanaugh J, Broer S (2004) Molecular cloning of mouse amino acid transport system B0, a neutral amino acid transporter related to Hartnup disorder. Biochem J 279:24467–24476
Burgos SA, Dai M, Cant JP (2010) Nutrient availability and lactogenic hormones regulate mammary protein synthesis through the mammalian target of rapamycin signaling pathway. J Dairy Sci 93:153–161
Bussolati O, Sala R, Astorri A, Rotoli BM, Dallasta V, Gazzola GC (1993) Characterization of amino acid transport in human endothelial cells. Am J Physiol Cell Physiol 265:C1006–C1014
Calvert DT, Shennan DB (1996) Evidence for an interaction between cationic and neutral amino acids at the blood-facing aspect of lactating rat mammary epithelium. J Dairy Res 63:25–33
Casey TM, Plaut K (2007) The role of glucocorticoids in secretory activation and milk secretion, a historical perspective. J Mam Gland Biol Neopl 12:293–304
Chillaron J, Roca R, Valencia A, Zorzano A, Palacı́n M (2001) Heterodimeric amino acid transporters: biochemistry, genetics and physiology. Am J Physiol Renal Physiol 281:F995–F1018
Chillarón J, Estévez R, Mora C, Wagner CA et al (1996) Obligatory amino acid exchange via systems bo,+-like and y+L-like. A tertiary active transport mechanism for renal reabsorption of cystine and dibasic amino acids. J Biol Chem 271(30):17761–17770
Clark JH, Derrig RG, Davis CL, Spires HR (1975) Metabolism of arginine and ornithine in the cow and rabbit mammary tissue. J Dairy Sci 58:1808–1813
Closs EI (1996) CATs, a family of three distinct mammalian cationic amino acid transporters. Amino Acids 11:193–200
Closs EI (2002) Expression, regulation and function of carrier proteins for cationic amino acids. Curr Opin Nephrol Hypertens 11:99–107
Closs EI, Albritton LM, Kim JW, Cunningham JM (1993) Identification of a low affinity, high capacity transporter of cationic amino acids in mouse liver. J Biol Chem 268:7538–7544
Dai ZL, Wu ZL, Wang JJ, Wang XQ, Jia SC, Bazer FW, Wu G (2014) Analysis of polyamines in biological samples by HPLC involving pre-column derivatization with o-phthalaldehyde and N-acetyl-l-cysteine. Amino Acids 46:1557–1564
Dave MH, Schulz N, Zecevic M, Wagner CA, Verrey F (2004) Expression of heteromeric amino acid transporters along the murine intestine. Am J Physiol 258:597–610
Davis TA, Nguyen HV, Garcia-Bravo R, Fiorotto ML, Jackson EM, Reeds PJ (1994a) Amino acid composition of the milk of some mammalian species changes with stage of lactation. Br J Nutr 72(6):845–853
Davis TA, Nguyen HV, Garcia-Bravo R, Fiorotto ML, Jackson EM, Lewis DS, Lee DR, Reeds PJ (1994b) Amino acid composition of human milk is not unique. J Nutr 124:1126–1132
DePeters EJ, Cant JP (1992) Nutritional factors influencing the nitrogen composition of bovine milk: a review. J Dairy Sci 75:2043–2070
DeSantiago S, Torres N, Suryawan A, Tovar AR, Hutson SM (1998) Regulation of branched-chain amino acid metabolism in the lactating rat. J Nutr 128:1165–1171
DeSantiago S, Ramırez I, Tovar AR, Ortíz N, Torres N, Bourges H (1999) Amino acid profiles in diet, plasma and human milk in Mexican rural lactating women. Nutr Res 19:1133–1143
Devés R, Boyd CA (1998) Transporters for cationic amino acids in animal cells: discovery, structure, and function. Physiol Rev 78(2):487–545
Devés R, Chavez P, Boyd CAR (1992) Identification of a new transport system (y+L) in human erythrocytes that recognizes lysine and leucine with high affinity. J Physiol 454:491–501
Emmanuel B, Kelly JJ (1984) Kinetics of methionine and choline and their incorporation into plasma lipids and milk components in lactating goats. J Dairy Sci 67:1912–1918
Erbersdobler HF, Trautwein E, Greulich HG (1984) Determinations of taurine in milk and infant formula diets. Eur J Pediatr 142(2):133–134
Frank JW, Escobar J, Suryawan A, Kimball SR, Nguyen HV, Jefferson LS, Davis TA (2005) Protein synthesis and translation initiation factor activation in neonatal pigs fed increasing levels of dietary protein. J Nutr 135(6):1374–1381
Frank JW, Escobar J, Suryawan A, Nguyen HV, Kimball SR, Jefferson LS, Davis TA (2006) Dietary protein and lactose increase translation initiation factor activation and tissue protein synthesis in neonatal pigs. Am J Physiol Endocrinol Metab 290:E225–E233
Ganguli MC, Speer VC, Ewan RC, Zimmerman DR (1971) Sulfur amino acid requirement of the lactating sow. J Anim Sci 33:394–400
Glass RD, Knox WE (1973) Arginase isozymes of rat mammary gland, liver, and other tissues. J Biol Chem 248:5785–5789
Guan X, Bequette BJ, Calder G, Ku PK, Ames KN, Trottier NL (2002) Amino acid availability affects amino acid transport and protein metabolism in the porcine mammary gland. J Nutr 132:1224–1234
Guan X, Pettigrew JE, Ku PK, Ames KN, Bequette BJ, Trottier NL (2004) Dietary protein concentration affects plasma arteriovenous difference of amino acids across the porcine mammary gland. J Anim Sci 82:2953–2963
Guinard J, Rulquin H (1994a) Effect of graded levels of duodenal infusions of casein on mammary uptake in lactating cows. J Dairy Sci 77:3304–3315
Guinard J, Rulquin H (1994b) Effects of graded amounts of duodenal infusions of lysine on the mammary uptake of major milk precursors in dairy cows. J Dairy Sci 77:3565–3576
Harper AE, Miller RH, Block KP (1984) Branched-chain amino acid metabolism. Annu Rev Nutr 4:409–454
Hayashi AA, Proud CG (2007) The rapid activation of protein synthesis by growth hormone requires signaling through mTOR. Am J Physiol Endocrinol Metab 292:E1647–E1655
Hayashi AA, Nones K, Roy NC, McNabb WC, Mackenzie DS, Pacheco D, McCoard S (2009) Initiation and elongation steps of mRNA translation are involved in the increase in milk protein yield caused by growth hormone administration during lactation. J Dairy Sci 92:1889–1899
Hundal HS, Taylor PM (2009) Amino acid transceptors: gate keepers of nutrient exchange and regulators of nutrient signaling. Am J Physiol Endocrinol Metab 296:E603–E613
Hurley WL, Wang H, Bryson JM, Shennan DB (2000) Lysine uptake by mammary gland tissue from lactating sows. J Anim Sci 78:391–395
Hyde R, Taylor PM, Hundal HS (2003) Amino acid transporters: roles in amino acid sensing and signalling in animal cells. Biochem J 373:1–18
Jackson SC, Bryson JM, Wang H, Hurley WL (2000) Cellular uptake of valine by lactating porcine mammary tissue. J Anim Sci 78:2927–2932
Johnson LR (1988) Regulation of gastrointestinal mucosal growth. Physiol Rev 68:456–502
Johnson TL, Fujimoto BAS, Flores R, Peterson DG (2010) Growth hormone alters lipid composition and increases the abundance of casein and lactalbumin mRNA in the MAC-T cell line. J Dairy Res 77:199–204
Jorgensen GN, Larson BL (1968) Conversion of phenylalanine to tyrosine in the bovine mammary secretory cell. Bioch Biophy Acta 165:121–126
Kakuda DK, Finley KD, Dionne VE, MacLeod CL (1993) Two distinct gene products mediate y+ type cationic amino acid transport in Xenopus oocytes and show different tissue expression patterns. Transgene 1:91–101
Kanai Y, Fukasawa Y, Cha SH et al (2000) Transport properties of a system y+L neutral and basic amino acid transporter. Insights into the mechanisms of substrate recognition. J Biol Chem 275:20787–20793
Kansal R, Kansal VR (1996) Discrimination of transport systems for l-tyrosine in mouse mammary gland: characterization of system T. Indian J Exp Biol 34:750–757
Kansal VK, Sharma R, Rehan G (2000) Characterization of anionic amino acid transport systems in mouse mammary gland. Indian J Exp Biol 38:1097–1103
Karunakaran S, Ramachandran S, Coothankandaswamy V et al (2011) SLC6A14 (ATB0,+) Protein, a Highly Concentrative and Broad Specific Amino Acid Transporter, Is a Novel and Effective Drug Target for Treatment of Estrogen Receptor-positive Breast Cancer. J Biol Chem 286(36):31830–31838
Kavanaugh MP (1993) Voltage dependence of facilitated arginine flux mediated by the system y+ basic amino acid transporter. Biochemistry 32:5781–5785
Kim SW, Wu G (2004) Dietary arginine supplementation enhances the growth of milk fed young pigs. J Nutr 134:625–630
Kim SW, Wu G (2009) Regulatory role for amino acids in mammary gland growth and milk synthesis. Amino Acids 37:89–95
Kim JW, Closs EI, Albritton LM, Cunningham JM (1991) Transport of cationic amino acids by the mouse ecotropic retrovirus receptor. Nature 352:725–728
Kim SW, Hurley WL, Han IK, Easter RA (1999) Changes in tissue composition associated with mammary gland growth during lactation in sows. J Anim Sci 77:2510–2516
Kim DK, Kanai Y, Chairoungdua A, Matsuo H, Cha SH, Endou H (2001) Expression cloning of a Na+-independent aromatic amino acid transporter with structural similarity to H+/monocarboxylate transporters. J Biol Chem 276:17221–17228
Kimball SR, Jefferson LS (2006) New functions for amino acids: effects on gene transcription and translation. Am J Clin Nutr 83:500S–507S
King RH, Toner MS, Dove H, Atwood Brown WG (1993) Response of first-litter sows to dietary protein level during lactation. J Anim CS Sci 71:2457–2463
Lacasse P, Prosser CG (2003) Mammary blood flow does not limit milk yield in lactating goats. J Dairy Sci 86:2094–2097
Lacasse P, Farr VC, Davis SR, Prosser CG (1996) Local secretion of nitric oxide and the control of mammary blood flow. J Dairy Sci 79:1369–1374
Lapierre H, Doepel L, Milne E, Lobley GE (2009) Responses in mammary and splanchnic metabolism to altered lysine supply in dairy cows. Animal 3:360–371
Lei J, Feng DY, Zhang YL, Dahanayaka S, Li XL, Yao K, Wang LL, Wu ZL, Dai ZL, Wu G (2012a) Regulation of leucine catabolism by metabolic fuels in mammary epithelial cells. Amino Acids 43:2179–2189
Lei J, Feng DY, Zhang YL, Zhao FQ, Wu ZL, San Gabriel A, Fujishima Y, Uneyama H, Wu G (2012b) Nutritional and regulatory role of branched-chain amino acids in lactation. Front Biosci 17:2725–2739
Lei J, Feng DY, Zhang YL, Dahanayaka S, Li XL, Yao K, Wang LL, Wu ZL, Dai ZL, Wu G (2013) Hormonal regulation of leucine catabolism in mammary epithelial cells. Amino Acids 45:531–541
Li P, Knabe DA, Kim SW, Lynch CJ, Hutson SM, Wu G (2009) Lactating porcine mammary tissue catabolizes branched-chain amino acids for glutamine and aspartate synthesis. J Nutr 139:1502–1509
Liang Z, Cho HT, Williams L et al (2011) Potential biomarker of L-type amino acid transporter 1 in breast cancer progression. Nucl Med Mol Imaging 45(2):93–102
López A, Torres N, Ortiz V, Aleman G, Hernandez-Pando R, Tovar AR (2006) Characterization and regulation of the gene expression of amino acid transport system A (SNAT2) in rat mammary gland. Am J Physiol Endocrinol Metab 291:E1059–E1066
Ma XM, Blenis J (2009) Molecular mechanisms of mTOR-mediated translational control. Nat Rev Mol Cell Biol 10(5):307–318
Mabjeesh SJ, Kyle CE, MacRae JC, Bequette BJ (2000) Lysine metabolism by the mammary gland of goats at two stages of lactation. J Dairy Sci 83:996–1003
Manjarín R, Steibel JP, Zamora V et al (2011) Transcript abundance of amino acid transporters, β-casein and α-lactalbumin in mammary tissue of peri-parturient, lactating and post-weaned sows. J Dairy Sci 94(7):3467–3476
Manjarín R, Zamora V, Wu G et al (2012) Effect of amino acids supply in reduced crude protein diets on performance, efficiency of mammary uptake, and transporter gene expression in lactating sows. J Anim Sci 90(9):3088–3100
Martínez-Lopez I, García C, Barber T, Vina JR, Miralles VJ (1998) The Lglutamate transporters GLAST (EAAT1) and GLT-1 (EAAT2): expression and regulation in rat lactating mammary gland. Mol Membr Biol 15:237–242
Mateo RD, Wu G, Bazer FW et al (2007) Dietary l-arginine supplementation enhances the reproductive performance of gilts. J Nutr 137:652–656
Mateo RD, Wu G, Moon HK, Carroll JA, Kim SW (2008) Effects of dietary arginine supplementation during gestation and lactation on the performance of lactating primiparous sows and nursing piglets. J Anim Sci 86:827–835
Matsumoto T, Nakamura E, Nakamura H, Hirota M, San Gabriel A, Nakamura KI, Chotechuang N, Wu G, Uneyama H, Torii K (2013) The production of free glutamate in milk requires the leucine transporter LAT1. Am J Physiol Cell Physiol 305:C623–C631
McDonald KK, Rouhani R, Handlogten ME, Block ER, Griffith OW, Allison RD, Kilberg MS (1997) Inhibition of endothelial cell amino acid transport system y+ by arginine analogs that inhibit nitric oxide synthase. Biochim Biophys Acta 1324:133–141
Meier C, Ristic Z, Klauser S, Verrey F (2002) Activation of system L heterodimeric amino acid exchangers by intracellular substrates. EMBO J 21:580–589
Meininger CJ, Wu G (2002) Regulation of endothelial cell proliferation by nitric oxide. Methods Enzymol 352:280–295
Mepham TB, Linzell JL (1966) A quantitative assessment of the contribution of individual plasma amino acids to the synthesis of milk proteins by the goat mammary gland. Biochem J 101:76–83
Mepham TB, Linzell JL (1967) Urea formation by the lactating goat mammary gland. Nature 214:507–508
Mezl VA, Knox WE (1977) Metabolism of arginine in lactating rat mammary gland. Biochem J 166(1):105–113
Millar ID, Calvert DT, Lomax MA, Shennan DB (1996) The mechanism of Lglutamate transport by lactating rat mammary tissue. Biochim Biophys Acta 1282:200–206
Millar ID, Calvert DT, Lomax MA, Shennan DB (1997) Substrate specificity of the mammary tissue anionic amino acid carrier operating in the cotransport and exchange modes. Biochim Biophys Acta 1326:92–102
Moshel Y, Rhoads RE, Barash I (2006) Role of amino acids in translational mechanisms governing milk protein synthesis in murine and ruminant mammary epithelial cells. J Cell Biochem 98:685–700
Motyl T, Ploszaj T, Wojtasik A, Kukulska W, Podgurniak M (1995) Polyamines in cow’s and sow’s milk. Comp Biochem Physiol B 111:427–433
Nawrath H, Wegener JW, Rupp J, Habermeier A, Closs EI (2000) Voltage dependence of l-arginine transport by hCAT-2A and hCAT-2B expressed in oocytes from Xenopus laevis. Am J Physiol Cell Physiol 279:C1336–C1344
Neville MC, Lobitz CJ, Ripoll EA, Tinney C (1980) The sites for alpha-aminoisobutyric acid uptake in normal mammary gland and ascites tumor cells. A comparative study of mouse tissues in vitro. J Biol Chem 255:7311–7316
Nichols JR, Schingoethe DJ, Brouk MJ, Piepenbrink MS (1998) Evaluation of corn distillers grains and ruminally protected HA lysine and methionine for lactating dairy cows. J Dairy Sci 81(2):482–491
Nielsen TT, Trottier NL, Stein HH, Bellaver C, Easter RA (2002) The effect of litter size and day of lactation on amino acid uptake by the porcine mammary glands. J Anim Sci 80(9):2402–2411
O’Quinn PR, Knabe DA, Wu G (2002) Arginine catabolism in the lactating porcine mammary tissue. J Anim Sci 80:467–474
Ogonowski AA, Kaesemeyer WH, Jin LM et al (2000) Effects of NO donors and synthase antagonists on endothelial cell uptake of l-arginine and superoxide production. Am J Physiol Cell Physiol 278:C136–C143
Oka T, Perry JW (1974) Arginase affects lactogenesis through its influence on the biosynthesis of spermidine. Nature (Lond) 250:660–661
Palacín M, Kanai Y (2004) The ancillary proteins of HATs: SLC3 family of amino acid transporters. Pflugers Arch 447:490–494
Palacín M, Estevez R, Bertran J, Zorzano A (1998) Molecular biology of mammalian amino acid transporters. Physiol Rev 78:969–1054
Pasantes-Morales H, López I, Ysunza A (1995) Taurine content in breast milk of Mexican women from urban and rural areas. Arch Med Res 26(1):47–52
Pau MY, Milner JA (1982) Effect of arginine deficiency on mammary gland development in the rat. J Nutr 112:1827–1833
Pérez-Laspiur JP, Trottier NL (2001) Effect of dietary Arginine supplementation and environmental temperature on sow lactation performance. Livest Prod Sci 70:159–165
Pérez-Laspiur JP, Burton JL, Weber PSD, Kirkwood RN, Trottier NL (2004) Short communication: AA transporters in porcine mammary gland during lactation. J Dairy Sci 87:3235–3237
Pérez-Laspiur JP, Burton JL, Weber PSD et al (2009) Dietary protein intake and stage of lactation differentially modulate AA transporter mRNA abundance in porcine mammary tissue. J Nutr 139:1677–1684
Pineda M, Fernández E, Torrents D et al (1999) Identification of a membrane protein (LAT-2) that co-expresses with 4F2hc an L type amino acid transport activity with broad specificity for small and large zwitterionic amino acids. J Biol Chem 274:19738–19744
Plaut KI, Kensinger RS, Griel LC, Kavanaugh JF (1989) Relationships among prolactin binding, prolactin concentrations in plasma and metabolic activity of the porcine mammary gland. J Anim Sci 67:1509–1519
Pollack PF, Koldousky O, Nishioka F (1992) Polyamines in human and rat milk and in infant formulas. Am J Clin Nutr 56:371–375
Prizant RL, Barash I (2008) Negative effects of the amino acids Lys, His, and Thr on S6K1 phosphorylation in mammary epithelial cells. J Cell Biochem 105:1038–1047
Prosser CG, Sankaran L, Hennighausen L, Topper YJ (1987) Comparison of the roles of insulin and insulin-like growth factor I in casein gene expression and in the development of α-lactalbumin and glucose transport activities in the mouse mammary epithelial cell. Endocrinology 120:1411–1416
Ramadan T, Camargo SM, Summa V et al (2006) Basolateral aromatic amino acid transporter TAT1 (Slc16a10) functions as an efflux pathway. J Cell Physiol 206:771–779
Ramirez I, De Santiago S, Tovar AR, Torres N (2001) Amino acid intake during lactation and amino acids of plasma and human milk. Adv Exp Med Biol 501:415–421
Razook-Hasan H, White DA, Mayer RJ (1982) Extensive degradation of newly synthesised casein in mammary explants in organ culture. Biochem J 202:133–138
Rezaei R, Knabe DA, Tekwe CD, Dahanayaka S, Ficken MD, Fielder SE, Eide SJ, Lovering SL, Wu G (2013a) Dietary supplementation with monosodium glutamate is safe and improves growth performance in postweaning pigs. Amino Acids 44:911–923
Rezaei R, Wang WW, Wu ZL, Dai ZL, Wang JJ, Wu G (2013b) Biochemical and physiological bases for utilization of dietary amino acids by young pigs. J Anim Sci Biotechnol 4:7
Rhoads RE, Nogalska EG (2007) Translational regulation of milk protein synthesis at secretory activation. J Mammary Gland Biol Neoplasia 12:283–292
Richert BT, Tokash MD, Goodband RD et al (1996) Valine requirement of the high-producing sow. J Anim Sci 74:1307–1313
Richert BT, Tokach MD, Goodband RD et al (1997) The effect of dietary lysine and valine fed during lactation on sow and litter performance. J Anim Sci 75:1853–1860
Roets E, Verbeke R, Massart-Leën AM, Peeters G (1974) Metabolism of [14C] citrulline in the perfused sheep and goat udder. Biochem J 144:435–446
Rogers QR (1976) The nutritional and metabolic effects of amino acid imbalances. In: Cole DJA, Boormann K, Buttery PJ, Lewis D, Neale RJ, Swan H (eds.) Protein metabolism and nutrition. Euro Assoc Anim Prod, 16, Butterworths, London, UK, pp 279–299
Rosen JM, Wyszomierski SL, Hadsell D (1999) Regulation of milk protein gene expression. Annu Rev Nutr 19:407–436
Rudolph MC, McManaman JL, Phang T et al (2007) Metabolic regulation in the lactating mammary gland: a lipid synthesizing machine. Physiol Genomics 28:323–336
Schneider R, Kirchgessner M, Paulicks BR, Schwarz FJ (1992) Eiweiss- und Aminosäurengehalte in der Sauenmilch bei unterschiedlicher Methioninversorgung. Mitteilung zum Bedarf laktierender Sauen an schwefelhaltigen Aminosäuren. J Anim Physiol Anim Nutr (Berl) 68:254–262
Segawa H, Fukasawa Y, Miyamoto K et al (1999) Identification and functional characterization of a Na+-independent neutral amino acid transporter with broad substrate selectivity. J Biol Chem 274:19745–19751
Sharma R, Kansal VK (1999) Characteristics of transport systems of l-alanine in mouse mammary gland and their regulation by lactogenic hormones: evidence for two broad spectrum systems. J Dairy Res 66:385–398
Sharma R, Kansal VK (2000) Heterogeneity of cationic amino acid transport systems in mouse mammary gland and their regulation by lactogenic hormones. J Dairy Res 67:21–30
Sharma R, Kansal VK (2001) Heterogeneity of transport systems for L-glutamine in mouse mammary gland. Indian J Biochem Biophys 38(4):241–248
Shennan DB, Peaker M (2000) Transport of milk constituents by the mammary gland. Physiol Rev 80:925–951
Shennan DB, Thomson J (2008) Inhibition of system L (LAT1/CD98hc) reduces the growth of cultured human breast cancer cells. Oncol Rep 20:885
Shennan DB, McNeillie SA, Jamison EA, Calvert DT (1994) Lysine transport in lactating rat mammary tissue: evidence for an interaction between cationic and neutral amino acids. Acta Physiol Scand 151:461–466
Shennan DB, Calvert DT, Travers MT, Kudo Y, Boyd CA (2002) A study of l-leucine, l phenylalanine and l-alanine transport in the perfused rat mammary gland: possible involvement of LAT1 and LAT2. Biochim Biophys Acta 1564:133–139
Shimomura Y, Obayashi M, Murakami T, Harris RA (2001) Regulation of branched-chain amino acid catabolism: nutritional and hormonal regulation of the expression of the branched-chain α-keto acid dehydrogenase kinase. Curr Opin Clin Nutr Metab Care 4:419–423
Sloan JL, Mager S (1999) Cloning and functional expression of a human Na+ and Cl– dependent neutral and cationic amino acid transporter B0,+. J Biol Chem 274:23740–23745
Sobrevia L, Cesare P, Yudilevich DL, Mann GE (1995) Diabetes-induced activation of system y+ and nitric oxide synthase in human endothelial cells: association with membrane hyperpolarization. J Physiol 489:183–192
Souba WW, Pacitti AJ (1992) How amino acids get into cells: mechanisms, models, menus and mediators. J Parenter Enteral Nutr 16:569–578
Sperandeo MP, Andria G, Sebastio G (2008) Lysinuric protein intolerance: update and extended mutation analysis of the SLC7A7 gene. H Mutation 29(1):14–21
Stipanuk MH (2007) Leucine and protein synthesis: mTOR and beyond. Nutr Rev 65:122–129
Taylor PM, Kaur S, Mackenzie B, Peter GJ (1996) Amino-acid-dependent modulation of amino acid transport in Xenopus laevis oocytes. J Exp Biol 199(Pt 4):923–931
Tokach MD, Goodband RD, Nelssen JL, Kats LJ (1993) Valine: a deficient amino acid in high lysine diets for the lactating sow. J Anim Sci 71(Suppl. 1):68 (Abstr.)
Torrents D, Estévez R, Pineda M et al (1998) Identification and characterization of a membrane protein (y + L amino acid transporter-1) that associates with 4F2hc to encode the amino acid transport activity y + L. A candidate gene for lysinuric protein intolerance. J Biol Chem 273(49):32437–32445
Tovar AR, Becerril E, Hernandez-Pando R et al (2001) Localization and expression of BCAT during pregnancy and lactation in the rat mammary gland. Am J Physiol Endocrinol Metab 280:E480–E488
Trottier N, Guan X (2000) Research paradigms behind amino acid requirements of the lactating sow: theory and future application. J Anim Sci 78:48–56
Trottier NL, Shipley CF, Easter RA (1997) Plasma amino acid uptake by the mammary gland of the lactating sow. J Anim Sci 75:1266–1278
Utsunomiya-Tate N, Endou H, Kanai Y (1996) Cloning and functional characterization of a system ASC-like Na+-dependent neutral amino acid transporter. Biochem J 271:14883–14890
Van Winkle LJ, Campione AL, Farrington BH (1990) Development of system Bo,+ and a broad-scope Na+-dependent transporter of zwitterionic amino acids in preimplantation mouse conceptuses. Biochim Biophys Acta 1025:225–233
Verbeke R, Peeters G, Massart-Leën AM, Cocquyt G (1968) Incorporation of DL-[2-14C]ornithine and DL-[5-14C]arginine in milk constituents by the isolated lactating sheep udder. Biochem J 106:719–724
Verbeke R, Roets E, Massart-Leën AM, Peeters G (1972) Metabolism of [U-14C]-l-threonine and [U-14C]-l-phenylalanine by the isolated perfused udder. J Dairy Res 39:239–250
Verma N, Kansal VK (1993) Characterization of the routes of methionine transport in mouse mammary glands. Indian J Med Res 98:297–304
Viña JR, Williamson DH (1981) Utilization of l-alanine and l-glutamine by lactating mammary gland of the rat. A role for l-alanine as a lipogenic precursor. Biochem J 196(3):757–762
Visek WJ (1985) Arginine and disease states. J Nutr 115:532–541
Wagner CA, Lang F, Bröer S (2001) Function and structure of heterodimeric amino acid transporters. Am J Physiol Cell Physiol 281(4):C1077–C1093
Wang X, Proud CG (2006) The mTOR pathway in the control of protein synthesis. Physiology (Bethesda) 21:362–369
Wang X, Proud CG (2009) Nutrient control of TORC1, a cell-cycle regulator. Trends Cell Biol 19:260–267
Wang H, Kavanaugh MP, North RA, Kabat D (1991) Cell-surface receptor for ecotropic murine retroviruses is a basic amino-acid transporter. Nature 352:729–731
Wang WW, Wu ZL, Dai ZL, Yang Y, Wang JJ, Wu G (2013) Glycine metabolism in animals and humans: implications for nutrition and health. Amino Acids 45:463–477
Wang WW, Dai ZL, Wu ZL, Lin G, Jia SC, Hu SD, Dahanayaka S, Wu G (2014a) Glycine is a nutritionally essential amino acid for maximal growth of milk-fed young pigs. Amino Acids 46:2037–2045
Wang L, Lin Y, Bian Y, Liu L, Shao L, Lin L, Qu B, Zhao F, Gao X, Li Q (2014b) Leucyl-tRNA synthetase regulates lactation and cell proliferation via mTOR signaling in dairy cow mammary epithelial cells. Int J Mol Sci 15:5952–5969
Watford M, Erbelding EJ, Smith EM (1986) Glutamine metabolism in rat small intestine: response to lactation. Biochem Soc Trans 14:1058–1059
Weekes TL, Luimes PH, Cant JP (2006) Responses to amino acid imbalances and deficiencies in lactating dairy cows. J Dairy Sci 89(6):2177–2187
White MF, Gazzola GC, Christensen HN (1982) Cationic amino acid transport into cultured animal cells. I. Influx into cultured human fibroblasts. J Biol Chem 257:4443–4449
Woodard MH, Dunn WA, Laine RO et al (1994) Plasma membrane clustering of system y+ (CAT-1) amino acid transporter as detected by immunohisto-chemistry. Am J Physiol Endocrinol Metab 266:E817–E824
Wu G (1997) Synthesis of citrulline and arginine from proline in enterocytes of postnatal pigs. Am J Physiol Gastrointest Liver Physiol 272:G1382–G1390
Wu G (1998) Intestinal mucosal amino acid catabolism. J Nutr 128:1249–1252
Wu G (2009) Amino acids: metabolism, functions, and nutrition. Amino Acids 37:1–17
Wu G (2010) Recent advances in swine amino acid nutrition. J Anim Sci Biotechnol 1:49–61
Wu G (2013a) Amino Acids: Biochemistry and Nutrition. CRC Press, Boca Raton
Wu G (2013b) Functional amino acids in nutrition and health. Amino Acids 45:407–411
Wu G (2014) Dietary requirements of synthesizable amino acids by animals: a paradigm shift in protein nutrition. J Anim Sci Biotechnol 5:34
Wu G, Knabe DA (1994) Free and protein-bound amino acids in sow’s colostrum and milk. J Nutr 124:415–424
Wu G, Meininger CJ (2000) Arginine nutrition and cardiovascular function. J Nutr 130:2626–2629
Wu G, Morris SM Jr (1998) Arginine metabolism: nitric oxide and beyond. Biochem J 336:1–17
Wu G, Haynes TE, Li H, Yan W, Meininger CJ (2001) Glutamine metabolism to glucosamine is necessary for glutamine inhibition of endothelial nitric oxide synthesis. Biochem J 353:245–252
Wu G, Knabe DA, Kim SW (2004) Arginine nutrition in neonatal pigs. J Nutr 134:2783S–2790S
Wu G, Bazer FW, Davis TA, Kim SW, Li P, Rhoads JM, Satterfield MC, Smith SB, Spencer TE, Yin YL (2009) Arginine metabolism and nutrition in growth, health and disease. Amino Acids 37:153–168
Wu G, Bazer FW, Burghardt RC, Johnson GA, Kim SW, Knabe DA, Li XL, Satterfield MC, Smith SB, Spencer TE (2010) Functional amino acids in swine nutrition and production. In: Doppenberg J, van der Aar P (eds) Dynamics in animal nutrition. Wageningen Academic Publishers, The Netherlands, pp 69–98
Wu G, Bazer FW, Burghardt RC, Johnson GA, Kim SW, Knabe DA, Li P, Li XL, McKnight JR, Satterfield MC, Spencer TE (2011a) Proline and hydroxyproline metabolism: implications for animal and human nutrition. Amino Acids 40:1053–1063
Wu G, Bazer FW, Johnson GA, Knabe DA, Burghardt RC, Spencer TE, Li XL, Wang JJ (2011b) Important roles for l-glutamine in swine nutrition and production. J Anim Sci 89:2017–2030
Wu G, Wu ZL, Dai ZL, Yang Y, Wang WW, Liu C, Wang B, Wang JJ, Yin YL (2013a) Dietary requirements of “nutritionally nonessential amino acids” by animals and humans. Amino Acids 44:1107–1113
Wu G, Bazer FW, Satterfield MC, Li XL, Wang XQ, Johnson GA, Burghardt RC, Dai ZL, Wang JJ, Wu ZL (2013b) Impacts of arginine nutrition on embryonic and fetal development in mammals. Amino Acids 45:241–256
Wu G, Bazer FW, Dai ZL, Li DF, Wang JJ, Wu ZL (2014) Amino acid nutrition in animals: protein synthesis and beyond. Annu Rev Anim Biosci 2:387–417
Yang H, Pettigrew JE, Johnston LJ, Shurson GC, Walker RD (2000) Lactational and subsequent reproductive responses of lactating sows to dietary lysine (protein) concentration. J Anim Sci 78:348–357
Yip MC, Knox WE (1972) Function of arginase in lactating mammary gland. Biochem J 127(5):893–899
Acknowledgments
This work was supported, in part, by funds from Texas A&M AgriLife Research (No. 8200) and National Research Initiative Competitive Grants from the Animal Growth and Nutrient Utilization Program (No. 2014-67015-21770) of the USDA National Institute of Food and Agriculture.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Manjarin, R., Bequette, B.J., Wu, G. et al. Linking our understanding of mammary gland metabolism to amino acid nutrition. Amino Acids 46, 2447–2462 (2014). https://doi.org/10.1007/s00726-014-1818-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00726-014-1818-8