Abstract
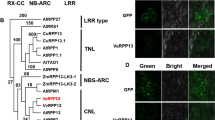
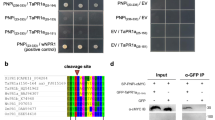
The NBS-LRR proteins encoded by the majority of R genes represent important intracellular receptors that directly or indirectly recognize pathogen effector proteins, which subsequently activate plant defense responses. In this study, a novel Plasmopara viticola-induced TIR-NBS-LRR gene, named VaRGA1, was cloned from leaf tissues of a highly downy mildew-resistant Vitis amurensis “Shuanghong” grapevine. The fluorescence signal of the VaRGA1-GFP fusion protein was clearly partitioned to the cytoplasm and nucleus. The expression of the VaRGA1 gene was strongly induced during early stages of infection by P. viticola, and was also significantly upregulated after drought and salt treatments. Accordingly, grapevine leaves transiently expressing the VaRGA1 gene manifested increased resistance to P. viticola, and the overexpression of the VaRGA1 gene in Nicotiana benthamiana conferred enhanced resistance to Phytophthora parasitica through the activation of salicylic acid (SA) signaling and phenylpropanoid pathways and could also increase tolerance to drought and salt stresses at the germination and vegetable growth stages. These findings indicate that the grapevine VaRGA1 gene may function as the immune and non-immune receptors against biotic and abiotic stresses and that there may be signaling overlap between biotic and abiotic responses.








Similar content being viewed by others
References
Aboul-Soud MA, Chen X, Kang JG, Yun BW, Raja MU, Malik SI, Loake GJ (2009) Activation tagging of ADR2 conveys a spreading lesion phenotype and resistance to biotrophic pathogens. New Phytol 183:1163–1175
Andreeva AV, Kutuzov MA (2001) Nuclear localization of the plant protein Ser/Thr phosphatase PP7. Mol Cell Biol Res Commun 4:345–352
Bai S et al (2012) Structure-function analysis of barley NLR immune receptor MLA10 reveals its cell compartment specific activity in cell death and disease resistance. PLoS Pathog 8:e1002752–e1002752
Barcelo J, Poschenrieder C, Andreu I, Gunse B (1986) Cadmium-induced decrease of water stress resistance in bush bean plants (Phaseolus vulgaris L. cv. contender) I. effects of Cd on water potential, relative water content, and cell wall elasticity. J Plant Physiol 125:17–25
Caplan JL, Mamillapalli P, Burch-Smith TM, Czymmek K, Dinesh-Kumar S (2008) Chloroplastic protein NRIP1 mediates innate immune receptor recognition of a viral effector. Cell 132:449–462
Césari S et al (2014) The NB-LRR proteins RGA4 and RGA5 interact functionally and physically to confer disease resistance. EMBO J 33:1941–1959
Cheng YT et al (2009) Nuclear pore complex component MOS7/Nup88 is required for innate immunity and nuclear accumulation of defense regulators in Arabidopsis. Plant Cell 21:2503–2516
Chini A, Grant JJ, Seki M, Shinozaki K, Loake GJ (2004) Drought tolerance established by enhanced expression of the CC–NBS–LRR gene, ADR1, requires salicylic acid, EDS1 and ABI1. Plant J 38:810–822
Ellis J, Dodds P, Pryor T (2000) Structure, function and evolution of plant disease resistance genes. Curr Opin Plant Biol 3:278–284
Faigón-Soverna A et al (2006) A constitutive shade-avoidance mutant implicates TIR-NBS-LRR proteins in Arabidopsis photomorphogenic development. Plant Cell 18:2919–2928
Feechan A et al (2013) Genetic dissection of a TIR-NB-LRR locus from the wild North American grapevine species Muscadinia rotundifolia identifies paralogous genes conferring resistance to major fungal and oomycete pathogens in cultivated grapevine. Plant J 76:661–674
Fossdal CG, Yaqoob N, Krokene P, Kvaalen H, Solheim H, Yakovlev IA (2012) Local and systemic changes in expression of resistance genes, NB-LRR genes and their putative microRNAs in Norway spruce after wounding and inoculation with the pathogen Ceratocystis polonica. BMC Plant Biol 12:105
Fujita M, Fujita Y, Noutoshi Y, Takahashi F, Narusaka Y, Yamaguchi-Shinozaki K, Shinozaki K (2006) Crosstalk between abiotic and biotic stress responses: a current view from the points of convergence in the stress signaling networks. Curr Opin Plant Biol 9:436–442
Gallois P, Marinho P (1995) Leaf disk transformation using Agrobacterium tumefaciens-expression of heterologous genes in tobacco. In Plant gene transfer and expression protocols, Methods in Molecular Biology, Vol. 49 (Jones, H., ed.), pp. 39-48. Totowa, NJ: Humana Press
Grant JJ, Chini A, Basu D, Loake GJ (2003) Targeted activation tagging of the Arabidopsis NBS-LRR gene, ADR1, conveys resistance to virulent pathogens. Mol Plant Microbe In 16:669–680
Gu K et al (2005) R gene expression induced by a type-III effector triggers disease resistance in rice. Nature 435:1122–1125
Gururani MA, Venkatesh J, Upadhyaya CP, Nookaraju A, Pandey SK, Park SW (2012) Plant disease resistance genes: current status and future directions. Physiol Mol Plant P 78:51–65
Hammond-Kosack KE, Jones J (1996) Resistance gene-dependent plant defense responses. Plant Cell 8:1773
Hammond-Kosack KE, Jones JD (1997) Plant disease resistance genes. Annu Rev Plant Biol 48:575–607
Heath RL, Packer L (1968) Photoperoxidation in isolated chloroplasts: I. kinetics and stoichiometry of fatty acid peroxidation. Arch Biochem Biophys 125:189–198
Heidrich K, Wirthmueller L, Tasset C, Pouzet C, Deslandes L, Parker JE (2011) Arabidopsis EDS1 connects pathogen effector recognition to cell compartment-specific immune responses. Science 334:1401–1404
Hewezi T, Mouzeyar S, Thion L, Rickauer M, Alibert G, Nicolas P, Kallerhoff J (2006) Antisense expression of a NBS-LRR sequence in sunflower (Helianthus annuus L.) and tobacco (Nicotiana tabacum L.): evidence for a dual role in plant development and fungal resistance. Transgenic Res 15:165–180
Iandolino A, da Silva FG, Lim H, Choi H, Williams L, Cook D (2004) High-quality RNA, cDNA, and derived EST libraries from grapevine (Vitis vinifera L.). Plant Mol Bio Rep 22:269–278
Igari K, Endo S, Ki H, Aida M, Sakakibara H, Kawasaki T, Tasaka M (2008) Constitutive activation of a CC-NB-LRR protein alters morphogenesis through the cytokinin pathway in Arabidopsis. Plant J 55:14–27
Inoue H et al (2013) Blast resistance of CC-NB-LRR protein Pb1 is mediated by WRKY45 through protein-protein interaction. P Natl Acad Sci USA 110:9577–9582
Jaillon O et al (2007) The grapevine genome sequence suggests ancestral hexaploidization in major angiosperm phyla. Nature 449:463–467
Jayakannan M, Bose J, Babourina O, Shabala S, Massart A, Poschenrieder C, Rengel Z (2015) The NPR1-dependent salicylic acid signalling pathway is pivotal for enhanced salt and oxidative stress tolerance in Arabidopsis. J Exp Bot 66:1865–1875
Jiang M, Zhang J (2001) Effect of abscisic acid on active oxygen species, antioxidative defence system and oxidative damage in leaves of maize seedlings. Plant Cell Physiol 42:1265–1273
Jones JD, Dangl JL (2006) The plant immune system. Nature 444:323–329
Kang G et al (2013) Exogenous salicylic acid enhances wheat drought tolerance by influence on the expression of genes related to ascorbate-glutathione cycle. Bio Plantarum 57:718–724
Kortekamp A, Welter L, Vogt S, Knoll A, Schwander F, Töpfer R, Zyprian E (2008) Identification, isolation and characterization of a CC-NBS-LRR candidate disease resistance gene family in grapevine. Mol Breeding 22:421–432
Lee S, Kim SG, Park CM (2010) Salicylic acid promotes seed germination under high salinity by modulating antioxidant activity in Arabidopsis. New Phytol 188:626–637
Levy SB, Marshall B (2004) Antibacterial resistance worldwide: causes, challenges and responses. Nat Med 10:S122–S129
Li X, Wu J, Yin L, Zhang Y, Qu J, Lu J (2015) Comparative transcriptome analysis reveals defense-related genes and pathways against downy mildew in Vitis amurensis grapevine. Plant Physiol Bioch 95:1–14
Liu J-J, Ekramoddoullah AK (2011) Genomic organization, induced expression and promoter activity of a resistance gene analog (PmTNL1) in western white pine (Pinus monticola). Planta 233:1041–1053
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2− ΔΔCT method. Methods 25:402–408
Malacarne G et al (2011) Resistance to Plasmopara viticola in a grapevine segregating population is associated with stilbenoid accumulation and with specific host transcriptional responses. BMC Plant Biol 11:114
Malacarne G et al (2012) Deconstruction of the (paleo) polyploid grapevine genome based on the analysis of transposition events involving NBS resistance genes. PloS one 7, e29762
Mutlu S, Atici Ö, Nalbantoglu B (2009) Effects of salicylic acid and salinity on apoplastic antioxidant enzymes in two wheat cultivars differing in salt tolerance. Biol Plantarum 53:334–338
Okuma E, Nozawa R, Murata Y, Miura K (2014) Accumulation of endogenous salicylic acid confers drought tolerance to Arabidopsis. Plant Signal Behav 9, e28085
Oldroyd GE, Staskawicz BJ (1998) Genetically engineered broad-spectrum disease resistance in tomato. P Natl Acad Sci USA 95:10300–10305
Pan X, Welti R, Wang X (2010) Quantitative analysis of major plant hormones in crude plant extracts by high-performance liquid chromatography-mass spectrometry. Nat Protoc 5:986–992
Radwan O et al (2008) Genetic diversity and genomic distribution of homologs encoding NBS-LRR disease resistance proteins in sunflower. Mol Genet Genomics 280:111–125
Santos-Rosa M, Poutaraud A, Merdinoglu D, Mestre P (2008) Development of a transient expression system in grapevine via agro-infiltration. Plant Cell Rep 27:1053–1063
Schwessinger B, Zipfel C (2008) News from the frontline: recent insights into PAMP-triggered immunity in plants. Curr Opin Plant Biol 11:389–395
Senaratna T, Touchell D, Bunn E, Dixon K (2000) Acetyl salicylic acid (Aspirin) and salicylic acid induce multiple stress tolerance in bean and tomato plants. Plant Growth Regu 30:157–161
Shen Q-H et al (2007) Nuclear activity of MLA immune receptors links isolate-specific and basal disease-resistance responses. Science 315:1098–1103
Stokes TL, Kunkel BN, Richards EJ (2002) Epigenetic variation in Arabidopsis disease resistance. Gene Dev 16:171–182
Szepesi Á, Csiszár J, Gémes K, Horváth E, Horváth F, Simon ML, Tari I (2009) Salicylic acid improves acclimation to salt stress by stimulating abscisic aldehyde oxidase activity and abscisic acid accumulation, and increases Na+ content in leaves without toxicity symptoms in Solanum lycopersicum L. J Plant Physiol 166:914–925
Takken FL, Albrecht M, Tameling WI (2006) Resistance proteins: molecular switches of plant defence. Curr Opin Plant Biol 9:383–390
Tameling WI, Joosten MH (2007) The diverse roles of NB-LRR proteins in plants. Physiological and Mol Plant Pathol 71:126–134
Tang X, Xie M, Kim YJ, Zhou J, Klessig DF, Martin GB (1999) Overexpression of Pto activates defense responses and confers broad resistance. Plant Cell 11:15–29
Tang J et al (2011) Semi-dominant mutations in the CC-NB-LRR-type R gene, NLS1, lead to constitutive activation of defense responses in rice. Plant J 66:996–1007
Wang Q et al (2011) Transcriptional programming and functional interactions within the Phytophthora sojae RXLR effector repertoire. Plant Cell 23:2064–2086
Wang X et al (2013) Characterization of a novel NBS-LRR gene involved in bacterial blight resistance in rice. Plant Mol Biol Rep 31:649–656
Wirthmueller L, Zhang Y, Jones JD, Parker JE (2007) Nuclear accumulation of the Arabidopsis immune receptor RPS4 is necessary for triggering EDS1-dependent defense. Curr Biol 17:2023–2029
Wu J, Zhang Y, Zhang H, Huang H, Folta KM, Lu J (2010) Whole genome wide expression profiles of Vitis amurensis grape responding to downy mildew by using Solexa sequencing technology. BMC Plant Biol 10:234
Yoshimura S et al (1998) Expression of Xa1, a bacterial blight-resistance gene in rice, is induced by bacterial inoculation. P Natl Acad Sci USA 95:1663–1668
Zhang L, Li Y, Lu W, Meng F, Wu CA, Guo X (2012) Cotton GhMKK5 affects disease resistance, induces HR-like cell death, and reduces the tolerance to salt and drought stress in transgenic Nicotianabenthamiana. J Exp Bot 63:3935-3951
Zipfel C (2008) Pattern-recognition receptors in plant innate immunity. Curr Opin Immunol 20:10–16
Zipfel C, Felix G (2005) Plants and animals: a different taste for microbes? Curr Opin Plant Biol 8:353–360
Acknowledgments
This work was supported by China Agriculture Research System (grant no. CARS-30-YZ-2), China Agricultural University Scientific Fund (grant no. 2012RC019), and National Natural Science Foundation of China (grant no. 3147175).
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling Editor: Hanns H. Kassemeyer
Electronic supplementary material
Below is the link to the electronic supplementary material.
Table S1
Primer sequences for qRT-PCR assay. (XLSX 9 kb)
Rights and permissions
About this article
Cite this article
Li, X., Zhang, Y., Yin, L. et al. Overexpression of pathogen-induced grapevine TIR-NB-LRR gene VaRGA1 enhances disease resistance and drought and salt tolerance in Nicotiana benthamiana . Protoplasma 254, 957–969 (2017). https://doi.org/10.1007/s00709-016-1005-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00709-016-1005-8