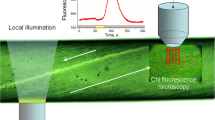
Abstract
Cytoplasmic streaming is essential for intracellular communications but its specific functions are not well known. In Chara corallina internodes, long-distance interactions mediated by cyclosis are clearly evident with microscopy-pulse amplitude modulation (PAM) fluorometer under application of localized light (LL) pulses to a remote cell region. Measurements of LL-induced profiles of chlorophyll fluorescence F′ at various distances from the LL source suggest that illuminated chloroplasts release into the streaming cytoplasm excess reducing equivalents that are entrained by the fluid flow and transiently reduce the intersystem electron carriers in chloroplasts of downstream shaded areas. The reducing equivalents propagate to distances up to 4.5 mm from the LL source, with the transport rate nearly equal to the velocity of liquid flow. The F′ transients disappeared after the arrest of streaming with cytochalasin D and reappeared upon its recovery in washed cells. The F′ responses to a distant LL were used as an indicator for the passage of cytosolic reductants across the analyzed cell area during measurements of cell surface pH (pHo) in intact and microperforated internodes. In microwounded cell regions, the LL-induced increase in F′ occurred synchronously with the increase in pHo, by contrast to a slight decrease in pHo observed prior to perforation. The results show that reducing agents transported with the cytoplasmic flow are involved in rapid pH changes on the surface of microinjured cells. A possibility is considered that cytoplasmic reductants are processed by stress-activated plasmalemmal NADPH oxidase carrying electrons to oxygen with the eventual H+ consumption on the outer cell side.







Similar content being viewed by others
Abbreviations
- AOI:
-
Area of inspection (area of interest)
- CW:
-
Cell wall
- LL:
-
Localized lighting (localized light)
- PFD:
-
Photon flux density
- pHo :
-
pH on the cell surface
References
Allahverdiyeva Y, Mustila H, Ermakova M, Bersanini L, Richaud P, Ajlani G, Battchikova N, Cournac L, Aro E-M (2013) Flavodiiron proteins Flv1 and Flv3 enable cyanobacterial growth and photosynthesis under fluctuating light. Proc Natl Acad Sci U S A 110:4111–4116
Allen NS, Allen RD (1978) Cytoplasmic streaming in green plants. Annu Rev Biophys Bioeng 7:497–526
Asada K (2006) Production and scavenging of reactive oxygen species in chloroplasts and their functions. Plant Physiol 141:391–396
Beilby MJ, Bisson MA (2012) pH banding in charophyte algae. In: Volkov AG (ed) Plant electrophysiology: methods and cell electrophysiology. Springer, Berlin, pp 247–271
Beilby MJ, Casanova MT (2014) The physiology of characean cells. Springer, Berlin
Bisson MA, Walker NA (1980) The Chara plasmalemma at high pH. Electrical measurements show rapid specific passive uniport of H+ or OH−. J Membr Biol 56:1–7
Bulychev AA, Dodonova SO (2011) Effects of cyclosis on chloroplast–cytoplasm interactions revealed with localized lighting in Characean cells at rest and after electrical excitation. Biochim Biophys Acta 1807:1221–1230
Bulychev AA, Kamzolkina NA (2006) Differential effects of plasma membrane electric excitation on H+ fluxes and photosynthesis in characean cells. Bioelectrochemistry 69:209–215
Bulychev AA, Komarova AV (2014) Proton flows across the plasma membrane in microperforated characean internodes: tonoplast injury and involvement of cytoplasmic streaming. Protoplasma 251:1481–1490
Bulychev AA, Komarova AV (2015) Photoinduction of cyclosis-mediated interactions between distant chloroplasts. Biochim Biophys Acta 1847:379–389
Bulychev AA, Komarova AV (2016) Influence of light on the apoplastic pH in microwounded cells of Chara corallina. Russ J Plant Physiol 63:46–53
Bulychev AA, Alova AV, Bibikova TN (2013a) Strong alkalinization of Chara cell surface in the area of cell wall incision as an early event in mechanoperception. Biochim Biophys Acta 1828:2359–2369
Bulychev AA, Alova AV, Rubin AB (2013b) Propagation of photoinduced signals with the cytoplasmic flow along characean internodes: evidence from changes in chloroplast fluorescence and surface pH. Eur Biophys J 42:441–453
Corneille S, Cournac L, Guedeney G, Havaux M, Peltier G (1998) Reduction of the plastoquinone pool by exogenous NADH and NADPH in higher plant chloroplasts: characterization of a NAD(P)H–plastoquinone oxidoreductase activity. Biochim Biophys Acta 1363:59–69
Dodonova SO, Bulychev AA (2011) Cyclosis-related asymmetry of chloroplast–plasma membrane interactions at the margins of illuminated area in Chara corallina cells. Protoplasma 248:737–749
Endo T, Mi H, Shikanai T, Asada K (1997) Donation of electrons to plastoquinone by NAD(P)H dehydrogenase and by ferredoxin–quinone reductase in spinach chloroplasts. Plant Cell Physiol 38:1272–1277
Eremin A, Bulychev AA, Hauser MJB (2013) Cyclosis-mediated transfer of H2O2 elicited by localized illumination of Chara cells and its relevance to the formation of pH bands. Protoplasma 250:1339–1349
Foissner I (1988) The relationship of echinate inclusions and coated vesicles on wound healing in Nitella flexilis (Characeae). Protoplasma 142:164–175
Foissner I, Wasteneys GO (2007) Wide-ranging effects of eight cytochalasins and latrunculin A and B on intracellular motility and actin filament reorganization in characean internodal cells. Plant Cell Physiol 48:585–597
Foissner I, Wasteneys GO (2012) The characean internodal cell as a model system for studying wound healing. J Microsc 247:10–22
Foyer CH, Noctor G (2009) Redox regulation in photosynthetic organisms: signaling, acclimation, and practical implications. Antioxid Redox Signal 11:861–905
Goldstein RE, van de Meent J-W (2015) A physical perspective on cytoplasmic streaming. Interface Focus 5:20150030
Hardham AR, Jones DA, Takemoto D (2007) Cytoskeleton and cell wall function in penetration resistance. Curr Opin Plant Biol 10:342–348
Heil M (2009) Damaged-self recognition in plant herbivore defence. Trends Plant Sci 14:356–363
Helman Y, Tchernov D, Reinhold L, Shibata M, Ogawa T, Schwarz R, Ohad I, Kaplan A (2003) Genes encoding A-type flavoproteins are essential for photoreduction of O2 in cyanobacteria. Curr Biol 13:230–235
Kalwarczyk T, Tabaka M, Holyst R (2012) Biologistics—diffusion coefficients for complete proteome of Escherichia coli. Bioinformatics 28:2971–2978
Klima A, Foissner I (2011) Actin-dependent deposition of putative endosomes and of endoplasmic reticulum during early stages of wound healing in characean internodal cells. Plant Biol 13:590–601
Krupenina NA, Bulychev AA (2007) Action potential in a plant cell lowers the light requirement for non-photochemical energy-dependent quenching of chlorophyll fluorescence. Biochim Biophys Acta 1767:781–788
Krupenina NA, Bulychev AA, Roelfsema MBG, Schreiber U (2008) Action potential in Chara cells intensifies spatial patterns of photosynthetic electron flow and non-photochemical quenching in parallel with inhibition of pH banding. Photochem Photobiol Sci 7:681–688
Leakey ADB, Scholes JD, Press MC (2005) Physiological and ecological significance of sunflecks for dipterocarp seedlings. J Exp Bot 56:469–482
Menzel D (1988) How do giant plant cells cope with injury?—the wound response in siphonous green algae. Protoplasma 144:73–91
Mitchell P (2011) Chemiosmotic coupling in oxidative and photosynthetic phosphorylation. Biochim Biophys Acta 1807:1507–1538
Monshausen GB, Bibikova TN, Weisenseel MH, Gilroy S (2009) Ca2+ regulates reactive oxygen species production and pH during mechanosensing in arabidopsis roots. Plant Cell 21:2341–2356
Neubauer C, Schreiber U (1989) Photochemical and non-photochemical quenching of chlorophyll fluorescence induced by hydrogen peroxide. Z Naturforsch 44c:262–270
Pearcy RW, Krall JP, Sassenrath-Cole GF (1996) Photosynthesis in fluctuating light environments. In: Baker NR (ed) Photosynthesis and the environment. Kluwer, Dordrecht, pp 321–346
Pickard WF (2003) The role of cytoplasmic streaming in symplastic transport. Plant Cell Environ 26:1–15
Rumeau D, Peltier G, Cournac L (2007) Chlororespiration and cyclic electron flow around PSI during photosynthesis and plant stress response. Plant Cell Environ 30:1041–1051
Shimmen T (2007) The sliding theory of cytoplasmic streaming: fifty years of progress. J Plant Res 120:31–43
Sommer A, Hoeftberger M, Hoepflinger MC, Schmalbrock S, Bulychev A, Foissner I (2015) Convoluted plasma membrane domains in the green alga Chara are depleted of microtubules and actin filaments. Plant Cell Physiol 56:1981–1996
Suzuki N, Miller G, Morales J, Shulaev V, Torres MA, Mittler R (2011) Respiratory burst oxidases: the engines of ROS signaling. Curr Opin Plant Biol 14:691–699
Taniguchi M, Miyake H (2012) Redox-shuttling between chloroplast and cytosol: integration of intra-chloroplast and extra-chloroplast metabolism. Curr Opin Plant Biol 15:252–260
Toko K, Chosa H, Yamafuji K (1985) Dissipative structure in the characeae: spatial pattern of proton flux as a dissipative structure in characean cells. J Theor Biol 114:125–175
Verchot-Lubicz J, Goldstein RE (2010) Cytoplasmic streaming enables the distribution of molecules and vesicles in large plant cells. Protoplasma 240:99–107
Acknowledgments
We are grateful to Prof. Ilse Foissner (University of Salzburg) for the gift of cytochalasin D and helpful discussion. This work was supported by the Russian Foundation for Basic Research, project no. 16-04-00318.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Handling Editor: Peter Nick
Rights and permissions
About this article
Cite this article
Bulychev, A.A., Komarova, A.V. Implication of long-distance cytoplasmic transport into dynamics of local pH on the surface of microinjured Chara cells. Protoplasma 254, 557–567 (2017). https://doi.org/10.1007/s00709-016-0975-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00709-016-0975-x