Abstract
Unconventional protein secretion (UPS) is a collective term for mechanisms by which cytosolic proteins that lack a signal peptide (“leaderless secretory proteins” (LSPs)) can gain access to the cell exterior. Numerous examples of UPS have been well documented in animal and yeast cells. In contrast, our understanding of the mechanism(s) and function of UPS in plants is very limited. This review evaluates the available literature on this subject. The apparent large numbers of LSPs in the plant secretome suggest that UPS also occurs in plants but is not a proof. Although the direct transport of LSPs across the plant plasma membrane (PM) has not yet been described, it is possible that as in other eukaryotes, exosomes may be released from plant cells through fusion of multivesicular bodies (MVBs) with the PM. In this way, LSPs, but also small RNAs (sRNAs), that are passively taken up from the cytosol into the intraluminal vesicles of MVBs, could reach the apoplast. Another possible mechanism is the recently discovered exocyst-positive organelle (EXPO), a double-membrane-bound compartment, distinct from autophagosomes, which appears to sequester LSPs.
Similar content being viewed by others
Introduction: conventional versus unconventional protein secretion
Conventional protein secretion (CPS) describes an intracellular transport process through which certain cytosolic proteins, by virtue of an N-terminal hydrophobic sequence of amino acids, first translocate into the lumen of the endoplasmic reticulum (ER) from where via vesicles they move to the Golgi apparatus and then to the plasma membrane (PM), where they are released into the apoplast (Lee et al. 2004; Osborne et al. 2005; Park and Jurgens 2012). Quite frequently, this term is often used to include the transport of soluble proteins to the vacuole, since they too traffic through the ER and Golgi apparatus before being segregated from apoplastic proteins in the trans-Golgi network (TGN), which is also the early endosome of plants (Contento and Bassham 2012). In this regard, it should be noted that there are numerous examples for vesicle-mediated protein transport in plants which start at the ER and circumvent the Golgi apparatus. Many of these relate to vacuolar protein transport and involve specialized ER transport vesicles (reviewed by De Marchis et al. 2013).
Conventionally, secreted soluble proteins have in common three characteristics: (1) They possess an N-terminal leader sequence, (2) they show some kind of posttranslational modification (glycosylation, phosphorylation, sulfation, etc.), and (3) their transport is blocked by application of brefeldin A (BFA). In the case of vacuolar proteins, the presence of an additional sequence-specific sorting determinant (Xiang et al. 2013; Pereira et al. 2014) would be a further characteristic. Properties (2) and (3) point to transport through the Golgi apparatus since this is where most of the glycoprotein processing enzymes reside (Schoberer and Strasser 2011; Schoberer et al. 2013) and where the targets for BFA action (ADP ribosylation factor-guanidine exchange factors (ARF-GEFs)) are located (Langhans et al. 2011; Naramoto et al. 2014; Doyle et al. 2015). In contrast, proteins that are released at the cell surface but lack a leader sequence show no posttranslational modifications and are unaffected by BFA fall into the category of unconventional protein secretion (UPS).
UPS in yeast and mammals: a brief update
Although by some there still remains lingering doubt that some of the proteins in question may simply reflect cytosolic proteins which have leaked out through cell lysis, the process of UPS now seems to enjoy the support of many leading yeast and mammalian cell biologists (e.g., Malhotra 2013; Zhang and Schekman 2013). UPS may occur through direct transit across the PM, of which there are two classes (see Fig. 1). Type I has been described by Rabouille et al. (2012) as “self-sustained protein translocation through lipid-induced oligomerization and membrane insertion,” and the best example of this is fibroblast growth factor 2 (FGF2). Recruitment of FGF2 to the inner lipid layer of the PM is mediated by phosphatidylinositol 4,5 biphosphate (PI(4,5)P2) and is stimulated by tyrosine (residue 82) phosphorylation (Steringer et al. 2012). FGF2 is fully folded when traversing the PM (Torrado et al. 2009), but its interaction with PI(4,5)P2 results in the oligomerization of FGF2, subsequently leading to the generation of a pore in the lipid bilayer. Oligomerization being dependent on the formation of disulfide bridges between the individual FGF2 monomers (Muller et al. 2015). Rabouille et al. (2012) have speculated that other proteins such as annexin A2, FGF1, and HIV Tat, which also bind to acidic phosphoinositides, may also cross the PM in the same way. Type II, known as “ABC-transporter-based secretion” translocates farnesylated peptides, e.g., α-factor in Saccharomyces cerevisiae and M-factor in Schizosaccharomyces pombe across the PM (Michaelis 1993). A number of acylated proteins appear to be released from parasitic protozoa by the same mechanism (Rabouille et al. 2012).
Overview of the major modes of unconventional protein secretion (UPS) in yeast and animal cells, including representative proteins for each pathway. Route a shows type I UPS, illustrating direct protein translocation across the plasma membrane. Route b presents shedding of microvesicles. Route c depicts the unconventional secretory pathway for acyl-CoA-binding protein 1 (Acb1) in the yeast Saccharomyces cerevisiae. Acb1 attaches to the surface of a novel compartment named the compartment of unconventional protein secretion (CUPS). As suggested by Malhotra (2013), CUPS has autophagosome-like characteristics and is formed under starvation conditions, but unlike true autophagosomes, it does not fuse with the vacuole. CUPS then releases vesicles from its outer membrane. These as yet unidentified vesicular carriers with Acb1 on their surfaces fuse with multivesicular bodies (MVBs), after which Acb1 is internalized into the intraluminal vesicles (ILVs) of MVBs. After fusion of the MVBs with the plasma membrane (PM), the ILVs are released as exosomes. Route d shows how autophagosomes may release their contents to the cell exterior by first fusing with MVBs to form a so-called amphisome. In this way, two sets of cargo molecules are set free. ER endoplasmic reticulum, ERES endoplasmic reticulum exit site, FGF2 fibroblast growth factor 2, IL-1β interleukin 1β, MATα mating factor α, sRNA small RNA
There are also cases where LSPs are believed to be indirectly secreted through fusion of some kind of organelle carrier with the PM. In reviews (e.g., Abrahamsen and Stenmark 2010), this is often depicted either as a multivesicular endosome (MVB), the intraluminal vesicles of which (containing the LSPs) are subsequently released at the cell surface as exosomes (see below for a further discussion), or a so-called amphisome which is a fusion hybrid of an autophagosome and a MVB. However, a fusion of amphisomes with the PM only occurs under pathological situations (Manjithaya and Subramani 2010); instead, they normally fuse with lysosomes (Klionsky 2007; Sanchez-Wandelmer and Reggiori 2013). Nevertheless, a number of articles have implicated a connection between autophagy and UPS (e.g., Duran et al. 2010; Manjithaya et al. 2010).
One of the most interesting examples for an MVB-mediated secretion is the case of the acyl-CoA-binding protein (Acb1) in yeast. This protein lacks a signal sequence, and its secretion is triggered by C- or N-starvation but does not occur in autophagy (atg) mutants. Thus, autophagosomes or more correctly their progenitors, the phagophore membranes, seem to be required for Acb1 secretion in yeast, but in keeping with the fact that the GTPase Ypt7 is not required for Acb1 secretion (Duran et al. 2010), these autophagic membranes do not fuse with the vacuole. Curiously, a Golgi tethering factor Grh1 is also involved in Acb1 secretion (Kinseth et al. 2007), and this protein relocates from ER exit sites and early Golgi membranes to a novel compartment termed compartment for unconventional protein secretion (CUPS) upon C-starvation (Bruns et al. 2011). Malhotra (2013) has proposed that Acb1 attaches to the cytosolic surface of Atg8-positive CUPs, which then release transport vesicles (single-membrane-bound in contrast to that of the CUPs) that subsequently fuse with a MVB. The Acb1 molecules then become internalized into the intraluminal vesicles (ILVs) of MVBs (see pathway c in Fig. 1).
Exosomes
Exosomes are of great interest in animal cell biology and the medical world (for two excellent recent reviews, see Colombo et al. 2014 and Kourembanas 2015). The reasons for this can be briefly summarized as follows. Antigen-presenting cells such as lymphocytes and dendritic cells secrete exosomes that carry peptide-loaded major histocompatibility complex (MHC) molecules which can stimulate T cell proliferation (Raposo et al. 1996). Exosomes appear to play crucial roles as carriers of prion proteins in neurodegenerative diseases (reviewed in Schneider and Simons 2013). They also participate in host–parasite interactions and act as modulators of the immune response (Schorey et al. 2015). However, perhaps the most important property of exosomes results from their ability to selective load mRNA and miRNA (taken up from the cytosol into the lumen of the invaginating ILV of the MVB), making them vectors of genetic exchange and communication between different cells in the human body (Valadi et al. 2007; Gibbings et al. 2009).
Since MVBs are traditionally regarded as endosomes, it has been asked whether MVBs which fuse with the PM and release exosomes are fundamentally different to those which deliver their contents to the lytic compartment of the cell. Preliminary observations suggest that this is likely: cholesterol-positive and cholesterol-negative MVBs seem to coexist in B lymphocytes, with only the cholesterol-enriched MVB being capable of fusing with the PM (Mobius et al. 2003). Another question centers on the molecular machinery responsible for ILV formation in the MVBs: Is it different for exosomal ILVs? Whereas ILVs destined for degradation in the lysosome are generally considered to be formed through the sequential action of the endosomal sorting complex required for transport (ESCRT) complexes (Hurley 2008; Raiborg and Stenmark 2009), there is some controversy as to mechanism(s) underlying the formation of exosomal ILVs. There is both evidence in favor of the participation of the ESCRT complexes (Baietti et al. 2012; Colombo et al. 2014), as well as against (Stuffers et al. 2009). In addition to tetraspanin proteins and cholesterol rafts (Simons and Raposo 2009), the lipid ceramide appears to play a crucial role (Trajkovic et al. 2008) in non-ESCRT mechanisms for exosomal ILV formation. Figure 2 presents a comparison of two possible mechanisms for ILV formation.
Mechanisms of intraluminal vesicle (ILV) formation in multivesicular bodies (MVBs). In a, invagination is achieved through lateral oligomerization of endosomal sorting complex required for transport (ESCRT) III complexes into lattices on the surface of the MVB. This causes the membrane to deform inwardly to produce an ILV. Through the action of the AAA-type ATPase Vps4, ESCRT III complexes are released from the MVB membrane. Another protein, Dos4, removes the ubiquitin tag, which is added to the cytosolic tails of receptor proteins as they are internalized at the plasma membrane (PM). These are transported downstream via endocytosis and are recognized by the first ESCRT complex (ESCRT 0) at the surface of the multivesicular endosome (for a detailed description of the ESCRT pathway, see Hurley 2008). b Alternative mechanism for ILV formation that does not involve receptor internalization into ILVs and relies on membrane deformation through local aggregations of cone-shaped ceramide molecules (see Trajkovic et al. 2008)
Equally important as the origin of exosomes is their fate. In order to interact with target cells, the exosomes have to be selectively bound at the recipient cell membrane and be internalized. In the case of T cells which are stimulated by MHC peptides, the interacting molecules have been identified as phosphatidyl serine receptors (Miyanishi et al. 2007) and leucocyte function-associated antigen-1 (LFA-1; (Nolte-'t Hoen et al. 2009)), but other examples need to be documented. It has been speculated that, like envelope viruses, exosomes could either fuse directly with the recipient cell membrane or be pino(phago)cytosed.
UPS in plants?
Examples for the direct translocation of proteins across the PM of plant cells remain to be discovered. A situation comparable to CUPS and the secretion of Acb1 or other acylated proteins has also not (yet) been described in plants. If one accepts the stringent criteria of Albenne et al. (2013), which in addition to the three characteristics of UPS mentioned above, also demands a positive immunolocalization in the apoplast, then we have at the moment only one verified case of UPS in plants. This is for Helja, a mannose-specific jacalin-related lectin in sunflower seedlings (Pinedo et al. 2012). However, published proteomic data indicate that the Arabidopsis secretome includes several tens of LSPs, which are described as belonging to either the cell wall or apoplastic proteome (Agrawal et al. 2010; see also Table 1 in Albenne et al. 2013). Similar values are also given in studies on a number of other plants (see Table 1 in Ding et al. 2012); Table 1 in Krause et al. 2013) and continue to be published (e.g., Lehtonen et al. 2014).
Obviously, the value of proteomics data is dependent on the purity of the organelle or subcellular fraction which is being examined (Albenne et al. 2013). This is particularly evident in the case of LSPs in the plant secretome, because their presence could merely reflect contamination with cytosolic proteins. Increased efforts to minimize or to exclude the consequences of cell damage have therefore been made, the methods most favored being the centrifugation of culture media, or the extraction of apoplastic fluid through vacuum infiltration followed by centrifugation (Agrawal et al. 2010). Combined with immunoblotting and enzymatic assays to monitor for the presence of bona fide cytosolic proteins (Alexandersson et al. 2013), these procedures are generally considered to deliver reliable data on LSPs (Krause et al. 2013). However, they are not entirely infallible: All cell cultures have dying and therefore lysed cells, and excised tissue inevitably exposes damaged cells at the cut surface. The recent paper of Guerra-Guimarães et al. (2015) puts the situation into a new perspective. These authors have performed a proteomic analysis of the apoplastic fluid extracted from Coffea arabica leaves. From the 116 identified proteins in the apoplastic fluid, only six were identified as putative LSPs, i.e., 5 % of the total proteins. However, because the estimated degree of contamination with cytoplasmic protein samples was also estimated at 5 % of the total leaf homogenates, serious doubt is cast on the true nature of the apoplastic proteins lacking a signal peptide.
Those who remain skeptical about the existence of LSPs in the plant secretome also point to the great variability in the numbers and types of LSP in the published data (Albenne et al. 2013). While we agree with the latter authors that the decisive proof that a LSP is present in the apoplast requires immunological confirmation in situ (e.g., through immunogold electron microscopy), it has to be mentioned that many secreted proteins are often in low copy number in the apoplast, and their expression is often restricted to specific developmental stages (Gupta and Deswal 2012). In this regard, it has been noted that many pathogen-related proteins, which are synthesized and released in response to pathogen attack or stress, e.g., chitinases and peroxidases, also lack a signal peptide (Ding et al. 2012; Krause et al. 2013).
Exosomes in plants?
Although many would consider the cell wall to be a major barrier to the movement of extracellular vesicles, there have been speculations about intercellular vesicle transport in both fungi (Nosanchuk et al. 2008) and higher plants (Regente et al. 2012). As yet, there is not much evidence in support of plant exosomes. The older ultrastructure literature contains numerous examples for possible MVB–PM fusion profiles (e.g., Gruner and Santore 1991; Robinson et al. 1996), and these are usually termed plasmalemmasomes, paramural bodies, or lomasomes. Whether such structures actually represent exocytic events or reflect an endocytic process (Herman and Lamb 1992) remains unresolved. Thus, it is currently not possible to estimate the frequency of MVB–PM fusion events in higher plant cells nor to judge what physiological role(s) the released exosomes might play.
In comparison to the situation in yeast and mammalian cells, not much is known about the biogenesis of MVBs and their ILVs in plants. MVBs seem to develop out of the TGN through a process of maturation in which ESCRT protein complexes are involved (Spitzer et al. 2009; Scheuring et al. 2011; Katsiarimpa et al. 2011; Gao et al. 2014). Data specifically related to the formation of exosomal ILVs in plants is not yet available.
Attempts have been made to isolate exosomes from the extracellular fluid of imbibed sunflower seeds, using a centrifugation procedure developed on animal tissues (Regente et al. 2009). A prominent protein in the exosome fraction was a 16 kDa lectin called agglutinin 1, which is similar to animal galectins. Interestingly, the galectins are adhesion-modulating molecules, which are involved in cancer and inflammation, and have also been detected in exosome preparations from mammals (Elola et al. 2007). Surprisingly, putative exosome fractions isolated from a variety of “edible plants” seem to be of potential therapeutic value as judged by their positive effects on the expression of an antioxidation gene and the production of anti-inflammatory cytokines when added to cultures of macrophages (Mu et al. 2014). However, the size of the putative exosomes (50–200 nm diameter) in these preparations is larger than the ILVs in typical plant MVBs (46.5 ± 9.5 nm diameter, C. Viotti personal communication), casting some doubt as to the authenticity/purity of the fractions in question. More convincing evidence for plant exosomes comes from recent studies on stigmatal exudates, which apparently contain up to 60 % LSPs (Rejon et al. 2013). These are also enriched in 30–60 nm diameter “nanovesicles” (Prado et al. 2014). Their role in pollen–stigma interactions is open to speculation.
Exosomes and the plant pathogen response?
Based on their studies of the basal resistance of Arabidopsis to Pseudomonas syringae, Wang et al. (2014) have suggested a possible correlation between MVB number and release of ILVs into the apoplast. These authors investigated the role of LYST-interacting protein 5 (LIP5), which stimulates suppressor of K+ transport growth defect 1 (SKD1), the AAA ATPase responsible for dissociation of ESCRT complexes from endosomes. Plants overexpressing a lip5-1 mutant gene are ten times as susceptible to the bacterial pathogen, and as judged by the number of fluorescent punctae with the MVB marker ARA6-GFP, cells from these plants formed fewer MVBs than wild-type cells did after bacterial infection. Wang et al. (2014) have also presented ultrastructural data in support of their claim that pathogen infection leads to an increase in MVB biogenesis and MVB–PM fusions. Infected wild-type plants had almost ten times as many MVBs and three times as many paramural bodies as infected lip5-1 mutant plants. Paramural bodies (see above) typically reveal large numbers of ILVs between the PM and the cell wall.
Filamentous pathogens (mainly oomycetes) invade leaves by moving through the cell wall and forming haustoria which protrude into cells, but leaving them intact (O'Connell and Panstruga 2006). Haustorial penetration triggers a number of structural and biochemical responses on the part of the host plant cells. Most significant is the development of a specialized domain of the PM surrounding the haustorium, termed the extrahaustorial membrane (EHM). The EHM lacks many of the typical PM resident proteins (Lu et al. 2012) but is characterized by the presence of REMORIN 1.3, a membrane raft marker protein (Bozkurt et al. 2014), and PDLP1, a plasmodesmata-associated protein involved in callose production (Caillaud et al. 2014). Other changes include a remodeling of the cytoskeleton, aggregation of the ER, and accumulation of organelles (mitochondria, peroxisomes, Golgi stacks, and secretory and endocytic vesicles) in the vicinity of the haustorium (Takemoto et al. 2003; Lu et al. 2012), all of which lead to a polarization of secretory activities (Meyer et al. 2009).
MVBs also accumulate in the vicinity of the penetration peg (An et al. 2006b; An et al. 2006a; Nielsen et al. 2012). One notes, however, that the MVBs depicted in the An et al. papers are large (diam ca. 1 μm, normal is around 500 nm), the density of ILVs is much higher than usually seen, and the ILVs themselves appear to be smaller than normal. Nevertheless, data strongly suggesting that MVBs do fuse with the haustoria (probably at the collar region of the penetration peg) has recently been published. Bozkurt et al. (2015) have shown that after oomycete infection, the GTPase RabG3c, which normally localizes to late endosomes and the tonoplast, is instead found at the EHM rather than the tonoplast. This might be explained by re-routing this Rab7 type GTPase from the vacuolar pathway to the endosome–PM recycling pathway. It could, however, also result from fusion of endosomes (MVBs) with the PM. Interestingly, upon infection, the receptor-like kinase BRI1 and its coreceptor BAK1, which are normally present on endosomes and over the whole surface of the PM, redistribute to the EHM and endosomes in the peri-haustorial region. A similar shift to the EHM and peri-haustorial localized endosomes was observed for the receptor for bacterial flagellin (FLS2) after its activation through exposure to its peptide ligand flg22. Thus, infection seems to cause a concentration of cell surface receptors and their recycling endosomes at the EHM and its underlying cytoplasm.
The PM-localized syntaxin PEN1 (=SYP121) is required for penetration resistance (Assaad et al. 2004) and normally cycles between the TGN and the PM. Upon infection, however, some of the PEN1-cycling membrane appears to reach the MVBs and, through internalization of the MVB membrane, enters the ILVs (Nielsen and Thordal-Christensen 2013). Green fluorescent protein (GFP)-tagged PEN1 is not only detected at the EHM but also in the matrix between the EHM and the cell wall of the haustorium (Meyer et al. 2009; Nielsen et al. 2012), suggesting that the matrix signal might represent secreted exosomes. As suggested by Nielsen and Thordal-Christensen (2013), these exosomes might play a decisive role in innate plant immunity. RNA silencing is well-documented to play a key role in plant defense, and small RNAs (sRNAs) can move from cell to cell via plasmodesmata in infected leaf tissue (Nunes and Dean 2012). However, the actual transport route of the sRNAs from the cytosol of the plant host into the haustorium cytosol of the invading pathogen has never been adequately explained. In mammalian cells, there is a large body of literature showing that exosomes act as agents of intercellular sRNA transfer (see above). It is therefore not unreasonable to expect that exosomes might act in a similar manner at the interface between the haustorium and host cell. The question of exosome internalization in mammalian systems has been addressed (see Record et al. 2011), but because of their size, exosomes cannot be taken up by clathrin-mediated endocytosis. Instead, both phagocytosis and direct fusion at the target cell PM seem to be possible. Since plants do not perform phagocytosis, the latter is the most likely mode of entry. A cartoon depicting this speculative and ingenious self-defense mechanism is given in Fig. 3.
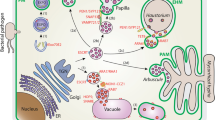
Illustration of how exosomes released from the infected plant cell at the point of haustorial infection may constitute a first line of defense against oomycete infection. a Schematic overview of the haustorium protruding into a leaf cell. The plant cell wall is partially digested at the contact site between the plasma membranes (PMs) of the host and the oomycete. The PM of the host cell in this region differentiates into the extrahaustorial membrane (EHM). Numerous organelles accumulate in the cytoplasm adjacent to the EHM, in particular multivesicular bodies (MVBs) and elements of the secretory pathway [endoplasmic reticulum (ER), Golgi stacks, and secretory vesicles (SVs)]. b Possible mechanism through which host cell small RNAs (sRNAs) may be transferred to the pathogen. The prominence of MVBs near the penetration peg and the recent demonstration that proteins characteristic of MVBs become incorporated into the EHM (Bozkurt et al. 2015) point to an enhanced fusion of the MVBs with the PM rather than with the vacuole, as would normally occur. Because the importance of RNA silencing in host–pathogen responses is already established (Nunes and Dean 2012), we think that the transfer mechanism for sRNAs is analogous to that involving exosomes in animal cells, i.e., entry of the sRNAs into the intraluminal vesicles (ILVs) of MVBs during the invagination phase, followed by release of the ILVs after MVB fusion with the EHM. Fusion of the ILVs with the PM of the haustorium would then release the sRNAs into the cytoplasm of the oomycete. G Golgi apparatus, HCW host cell wall, HPM host plasma membrane, Mt mitochondrion, OCW oomycete cell wall, OPM oomycete plasma membrane
Exocyst-positive organelles and UPS in plants
The exocyst complex
Transport vesicles can only fuse with their target organelles if the membranes of the vesicle and target have the right combination of soluble NSF attachment protein receptors (SNAREs) (Jahn and Scheller 2006). Helping the vesicle (v), SNAREs that find their t-SNARE partner are the so-called tethering factors (Chia and Gleeson 2014). These not only physically link the vesicle to its target membrane, but together with SM (Sec1/Munc18) proteins, they also regulate the assembly of the SNARE complexes (Hong and Lev 2014). There are two major subclasses of tethering factors: (a) extended coiled-coil proteins, used for long-distance (⑦ 200 nm) capturing and (b) compact multisubunit complexes, for short-range (⑥ 30 nm) interactions (Gillingham and Munro 2003; Brocker et al. 2010). Both types are evolutionary highly conserved and are distributed throughout the secretory and endocytic pathways (Yu and Hughson 2010; Wideman et al. 2014). The tethering factor called exocyst was originally discovered in a yeast secretion mutant screen and was shown to be essential for exocytosis (Novick et al. 1980; TerBush et al. 1996). It was later identified in mammals and Drosophila (Ting et al. 1995; Murthy et al. 2003) and also in plants (Hala et al. 2008).
Exocyst has been isolated as an octameric complex from yeast and consists of eight proteins: Sec3, Sec5, Sec6, Sec8, Sec10, Sec15, Exo70, and Exo84 (Terbush et al. 2001). These proteins have little sequence similarity to one another but share the following structural features: rod-shaped and tandem helical bundles each composed ofthree to five α-helices (Munson and Novick 2006; Heider and Munson 2012). This leads to a Y-shaped macromolecular structure (30 × 13 nm stalk; two 6 × 15 nm arms), which can even be visualized as such in the electron microscope (Hsu et al. 1999). Interactions between individual exocyst subunits have been studied through a variety of methods leading to the conclusion that most subunits are capable of interacting with numerous other subunits (Munson and Novick 2006). Two of the eight exocyst subunits are responsible for the initial attachment to the PM at the future site of exocytosis: Sec3 and Exo70. Both interact with PI(4,5)P2 in the inner lipid layer of the PM via polybasic sequences, located either at the N terminus (for Sec3) or at the C terminus (for Exo70) (He et al. 2007; Liu et al. 2007; Zhang et al. 2008). This appears to be also true for mammals (He and Guo 2009) and plants (L. Jiang and Y. Ding, unpublished data).
Plant exocyst
Whereas in yeast and mammals there are only single copies of each exocyst subunit genes, in plants, gene duplication has taken place several times during the course of evolution leading to multiple paralogs (Zhang et al. 2010; Cvrckova et al. 2012). Thus, in Arabidopsis, only Sec6 and Sec8 are present as single copy genes, whereas there are two each for Sec3, Sec5, Sec10, and Sec15; three for Exo84; and 23 for Ex70 (see Table 1 in Cvrckova et al. 2012 and Table 1 in Vukasinovic et al. 2014). In rice, there are even 47 paralogs for Exo70. There is little sequence similarity between the various Exo70 paralogs, especially in the N-terminal 300 amino acids (Cvrckova et al. 2012). This large diversity of exocyst subunits lead Cvrckova et al. (2012) to suggest that plants may have several different exocyst complexes corresponding to the greater diversification of endomembrane structure and function in plants as well as developmental stage differences in expression.
The exocyst complex in plants has been shown to participate in conventional exocytic events during normal cell wall growth (Kulich et al. 2015), cell plate formation (Zhang et al. 2013; Rybak et al. 2014), compatible pollen responses in stigmatic papillae (Safavian et al. 2014), and in response to pathogen attack (Pecenkova et al. 2011; Zhao et al. 2015). As in animal cells (see review by Heider and Munson 2012), the exocyst complex is also required for autophagosome formation in plants (Tzfadia and Galili 2013). In particular, the exocyst subunit Exo70B1 appears to be specifically required for autophagosome formation in Arabidopsis (Kulich et al. 2013).
EXPOs: plant-specific compartments for UPS?
EXPO is a novel double-membrane-bound organelle, which was initially discovered by transiently expressing AtExo70E2-(X)FP in protoplasts from Arabidopsis suspension-cultured cells (Wang et al. 2010). The fluorescent signal coming from this construct was punctate, mainly located at the PM but also within the cytosol, and did not overlap with fluorescent signals from standard marker constructs for the major endomembrane compartments (tonoplast, the Golgi apparatus, the TGN/early endosome, the prevacuolar compartment/late endosome) and the PM itself. Treatments with inhibitors of the secretory (brefeldin A; concanamycin A) and endocytic (wortmannin) pathways did not perturb the pattern of AtExo70E2-(X)FP labeling pointing to the unusual nature of the Exo70E2-positive membranes. That the fluorescent signals correctly reflected the intact AtExo70E2-(X)FP construct was demonstrated with antibodies against the N terminus of Exo70E2 as well as GFP antibodies. Poulsen et al. (2014) have independently confirmed these results using Nicotiana benthamiana leaves for the transient expression of AtExo70E2-(X)FP.
It has been suggested that EXPOs are artifacts of overexpression caused by the use of the cauliflower mosaic virus (CaMV) 35S promoter (see above and Zarsky et al. 2013). Speaking against this are the facts that (a) EXPO can be detected by immunofluorescence and immunogold electron microscopy in wild-type cells (Wang et al. 2010; Ding et al. 2014) and (b) similar densities of fluorescent Exo70E2 punctae are visible in Arabidopsis protoplasts expressing Exo70E2-GFP under the control of the endogenous Exo70E2 promoter (Ding et al. 2014). Nevertheless, it cannot be disputed that the strength and number of Exo70E2-GFP signals is higher in transient expression systems as compared to stable transgenic plants expressing AtExo70E2. Also, the strength of Exo70E2 signals in root cap cells of stable transgenic Arabidopsis plants is weaker when using the endogenous promoter (Lin et al. 2015). Thus, EXPOs are not artifacts, but their numbers are influenced by the degree of AtExo70E2 expression.
Electron micrographs of fusion profiles of EXPO with the PM (see Fig. 4 and also Fig. 8 in Wang et al. 2010; Figs. 3 in Ding et al. 2012, 2014) strongly suggest that EXPO have an exocytic function, especially since they do not label with the endocytic tracer dye FM4-64 (Wang et al. 2010). Wang et al. (2010) presented evidence pointing to a role for EXPO in UPS, by showing that a GFP-tagged version of the LSP S-adenosylmethionine synthetase 2 (SAMS2, At4G01850), one of four SAM enzymes involved in lignin biosynthesis, slowly accumulated as fluorescent punctae in the cytosol. These punctae colocalized with coexpressed Exo70E2-mRFP. Although SAMS2 could not be detected outside of the cell, Wang et al. (2010) did provide evidence for the presence of Exo70E2 in the culture medium. Control immunoblotting with antibodies against a number of soluble and integral membrane proteins ruled out the possibility that the extracellular Exo70E2 was a result of cell breakage. When EXPO fuses with the PM, it releases a single-membrane-bound vesicle into the interface between the PM and the cell wall. Exo70E2-GFP-positive vesicles have been detected at this location by immunogold electron microscopy but were also detected by fluorescence microscopy as punctae attached to the inner surface of the cell wall in cells subjected to plasmolysis (see Figs. 9 and 14, Wang et al. 2010).
Electron microscopy images of EXPOs in sections prepared from high-pressure frozen samples. a An EXPO in cross section in the cytoplasm; note the two boundary membranes (the arrows indicate the PM). The luminal content has the same appearance as the surrounding cytosol. b An EXPO captured just after fusion with the plasma membrane (indicated by the arrows). A single-membrane-bound vesicle exosome has been released. Magnification bars = 500 nm
A role for EXPO in the synthesis and secretion of arabinogalactan proteins has been recently published by Poulsen et al. (2014, 2015). These authors have identified EXPOs in leaf epidermal cells of N. benthamiana by transiently expressing AtExo70E2. Unexpectedly, three O-galactosyl transferases (AtGALT31A, AtGALT29A, AtGALT14A) localize to EXPOs as well as to Golgi stacks. In fact, it was estimated that 80 % of AtGALT31A was associated with EXPOs. In addition to galactosyl transferases, an apyrase and two UDP-glucuronate epimerases have also been detected in N. benthamiana leaf EXPOs (Poulsen et al. 2015). This data is difficult to understand, since O-galactosyl transferases are typically predicted to be type II membrane proteins (Herta Steinkellner, personal communication). So, how these get inserted into the membrane of EXPOs remains a mystery. Secondly, it is difficult to evaluate these results in terms of UPS, since as far as we are aware, there are no arabinogalactan proteins that lack a leader sequence.
Interestingly, EXPOs cannot be induced in plant cells by transiently expressing mammalian or yeast Exo70 homologs. However, EXPO-like structures are formed in animal cells when AtExo70-GFP is overexpressed. Overexpression of human Exo70-GFP in an animal cell line also gave rise to large fluorescent punctae, but these did not overlap with the signals for AtExo70E2-mRFP when coexpressed (Ding et al. 2014). Zhao et al. (2013) have shown that oligomerization of mammalian Exo70 can cause membranes in vitro to curve, ultimately forming tubules. This is a property of Exo70 which might lie behind the generation of EXPO-like structures in mammalian cells and which might contribute to their increased presence in plant cells upon overexpression of AtExo70E2.
EXPO and autophagosomes: distinct but related organelles
Zarsky et al. (2013) have established that in Arabidopsis leaves Exo70B1, but not Exo70A1, is associated with autophagosomes but have suggested that EXPOs may just be a special form of autophagosome. However, in contrast to the Exo70B1-labeled autophagosomes, which become sequestered in the central vacuole, a fusion of EXPO with the tonoplast was never observed. Instead. EXPO was consistently seen to fuse with the PM (Wang et al. 2010). Nevertheless, because of the great morphological similarity between autophagosomes and EXPO, it is tempting to speculate that the two compartments may at least have a common origin. In a recent attempt to shed light on this matter, Lin et al. (2015) have performed a thorough investigation on the distribution of fluorescent signals for ATG8e/f and Exo70E2 in wild-type suspension cultured cells and root tissue cells of Arabidopsis plants growing under normal and starvation-induced conditions. Immunofluorescence studies on normal growing stable transgenic plants expressing either Exo70E2-GFP or YFP-ATG8 clearly demonstrated no colocalization of EXPO and autophagosomal markers (see Fig. 5a, b). Upon induction of autophagy in the presence of concanmycin A, which prevents vacuolar acidification, autophagic bodies accumulate in the vacuole lumen. These are normally regarded as being as residual autophagosomes after their fusion with the tonoplast and typically are still marked by YFP-ATG8. However, in double transgenic plants subjected to autophagy, both EXPO and autophagosomal marker signals gradually accumulate in the vacuole, and these fluorescent signals show a high degree of overlap (see Fig. 5c, d). This suggests that EXPO and autophagosomes may indeed be related to one another. Lin et al. (2015) have speculated that the colocalization of marker signals in the vacuole after induction of autophagy might result from the heterotypic fusion of EXPO and autophagosomes, either before or after fusion with the tonoplast. The situation could, however, arise by EXPO preferentially recruiting ATG8 upon the onset of autophagy. This is supported by the observation that whereas membrane recruitment of ATG8 increased during autophagic induction, that of Exo70E2 did not.
Detection of EXPO and autophagosomes by fluorescence microscopy in Arabidopsis root cells. a Confocal laser scanning microscopy image from a stable transgenic plant expressing the EXPO marker AtExo70E2-GFP (indicated by arrowheads) and b from a stable transgenic plant expressing AtExo70E2-GFP but subsequently processed for immunofluorescence microscopy with ATG8e antibodies. EXPOs, as marked by Exo70E2-GFP, appear as individual green fluorescent punctae (indicated by arrowheads). Autophagosomes, as marked by ATG8e antibodies, appear as magenta fluorescent punctae (indicated by arrows) and are clearly separate from the EXPO signals. c, d Behavior of EXPOs and autophagosomes in roots induced to undergo starvation (through application of BTH) and in the presence of concanamycin A (to prevent proteolysis in the vacuole). In order to visualize the position of the tonoplast, the cells were stained with the styryl dye FM4-64, which is taken up at the PM and transported via endocytosis to the tonoplast (see Lin et al. 2015 for experimental details). Punctate fluorescent signals inside the vacuole lumen are observed for both EXPO (AtExo70E2) and autophagosome (ATG8e) markers. Magnification bars = 10 μm
Conclusions and future issues
-
1.
There is circumstantial evidence in support of UPS occurring in plants, but we still lack unequivocal hard biochemical and genetic evidence in its favor. Recurrent publication of LSPs in the plant secretome will not change this situation. In-depth studies of the exocytosis of individual apoplastic LSPs are now required.
-
2.
In general, multivesicular body (MVB)–PM fusions in plant cells occur infrequently, which makes it difficult to analyze exosome function. However, the accumulation of MVBs around the infection peg of invading oomycete haustoria presents a more exploitable situation. High-quality cryo-electron microscopy, including three-dimensional transmission electron microscopy tomography, is needed to confirm the release of exosomes into the joint apoplastic space between the extrahaustorial membrane and haustorial PM. However, identifying cargo in the interiors of exosomes represents a considerable technical challenge.
-
3.
With regard to the recently discovered EXPO, there are a number of avenues open for future research. First, the availability of specific AtExo70E2 antibodies makes an immunoisolation of this novel compartment possible, which could lead to a proteomic analysis and therefore identification of cargo and membrane proteins. Such studies will not only confirm EXPO function in UPS but will also give clues as to the biogenesis of this organelle and its relationship to autophagosomes.
-
4.
Superresolution live-cell imaging of stable transgenic plants expressing AtExo70E2-GFP can be expected to deliver crucial information on the dynamics of EXPO function and possibly identify smaller transport vesicles that might also participate in EXPO formation.
-
5.
The use of exo70e2 mutants will likely provide decisive information on the requirements of Exo70E2 for EXPO formation and EXPO-mediated UPS. Together with Exo70E2 transgenic plants, these mutants will also enable studies of the possible roles of Exo70E2/EXPO in plant defense.
Abbreviations
- Acb1:
-
Acyl-CoA-binding protein 1
- BFA:
-
Brefeldin A
- CPS:
-
Conventional protein secretion
- CUPS:
-
Compartment of unconventional protein secretion
- EHM:
-
Extrahaustorial membrane
- ER:
-
Endoplasmic reticulum
- ESCRT:
-
Endosomal sorting complex required for transport
- EXPO:
-
Exocyst-positive organelle
- ILV:
-
Intraluminal vesicle
- LSP:
-
Leaderless secretory protein
- MVB:
-
Multivesicular body
- PM:
-
Plasma membrane
- SNARE:
-
Soluble NSF attachment protein receptor
- TGN:
-
Trans-Golgi network
- UPS:
-
Unconventional protein secretion
References
Abrahamsen H, Stenmark H (2010) Protein secretion: unconventional exit by exophagy. Curr Biol 20:R415–418
Agrawal GK, Jwa NS, Lebrun MH, Job D, Rakwal R (2010) Plant secretome: unlocking secrets of the secreted proteins. Proteomics 10:799–827
Albenne C, Canut H, Jamet E (2013) Plant cell wall proteomics: the leadership of Arabidopsis thaliana. Front Plant Sci 4:111
Alexandersson E, Ali A, Resjo S, Andreasson E (2013) Plant secretome proteomics. Front Plant Sci 4:9
An Q, Ehlers K, Kogel KH, van Bel AJ, Huckelhoven R (2006a) Multivesicular compartments proliferate in susceptible and resistant MLA12-barley leaves in response to infection by the biotrophic powdery mildew fungus. New Phytol 172:563–576
An Q, Huckelhoven R, Kogel KH, van Bel AJ (2006b) Multivesicular bodies participate in a cell wall-associated defence response in barley leaves attacked by the pathogenic powdery mildew fungus. Cell Microbiol 8:1009–1019
Assaad FF et al (2004) The PEN1 syntaxin defines a novel cellular compartment upon fungal attack and is required for the timely assembly of papillae. Mol Biol Cell 15:5118–5129
Baietti MF, Zhang Z, Mortier E, Melchior A, Degeest G, Geeraerts A, Ivarsson Y, Depoortere F, Coomans C, Vermeiren E, Zimmermann P, David G (2012) Syndecan-syntenin-ALIX regulates the biogenesis of exosomes. Nat Cell Biol 14:677–685
Bozkurt TO, Belhaj K, Dagdas YF, Chaparro-Garcia A, Wu CH, Cano LM, Kamoun S (2015) Rerouting of plant late endocytic trafficking toward a pathogen interface. Traffic 16:204–226
Bozkurt TO, Richardson A, Dagdas YF, Mongrand S, Kamoun S, Raffaele S (2014) The plant membrane-associated REMORIN1.3 accumulates in discrete perihaustorial domains and enhances susceptibility to Phytophthora infestans. Plant Physiol 165:1005–1018
Brocker C, Engelbrecht-Vandre S, Ungermann C (2010) Multisubunit tethering complexes and their role in membrane fusion. Curr Biol 20:R943–952
Bruns C, McCaffery JM, Curwin AJ, Duran JM, Malhotra V (2011) Biogenesis of a novel compartment for autophagosome-mediated unconventional protein secretion. J Cell Biol 195:979–992
Caillaud MC et al (2014) The plasmodesmal protein PDLP1 localises to haustoria-associated membranes during downy mildew infection and regulates callose deposition. Plos Pathog 10:e1004496
Chia PZ, Gleeson PA (2014) Membrane tethering. F1000prime reports 6:74
Colombo M, Raposo G, Thery C (2014) Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu Rev Cell Dev Biol 30:255–289
Contento AL, Bassham DC (2012) Structure and function of endosomes in plant cells. J Cell Sci 125:3511–3518
Cvrckova F, Grunt M, Bezvoda R, Hala M, Kulich I, Rawat A, Zarsky V (2012) Evolution of the land plant exocyst complexes. Front Plant Sci 3:159
De Marchis F, Bellucci M, Pompa A (2013) Traffic of human alpha-mannosidase in plant cells suggests the presence of a new endoplasmic reticulum-to-vacuole pathway without involving the Golgi complex. Plant Physiol 161:1769–1782
Ding Y et al (2014) Exo70E2 is essential for exocyst subunit recruitment and EXPO formation in both plants and animals. Mol Biol Cell 25:412–426
Ding Y, Wang J, Wang J, Stierhof YD, Robinson DG, Jiang L (2012) Unconventional protein secretion. Trends Plant Sci 17:606–615
Doyle SM et al (2015) An early secretory pathway mediated by GNOM-LIKE 1 and GNOM is essential for basal polarity establishment in Arabidopsis thaliana. Proc Natl Acad Sci U S A 112:E806–815
Duran JM, Anjard C, Stefan C, Loomis WF, Malhotra V (2010) Unconventional secretion of Acb1 is mediated by autophagosomes. J Cell Biol 188:527–536
Elola MT, Wolfenstein-Todel C, Troncoso MF, Vasta GR, Rabinovich GA (2007) Galectins: matricellular glycan-binding proteins linking cell adhesion, migration, and survival. Cell Mol Life Sci 64:1679–1700
Gao C, Luo M, Zhao Q, Yang R, Cui Y, Zeng Y, Xia J, Jiang L (2014) A unique plant ESCRT component, FREE1, regulates multivesicular body protein sorting and plant growth. Curr Biol 24:2556–2563
Gibbings DJ, Ciaudo C, Erhardt M, Voinnet O (2009) Multivesicular bodies associate with components of miRNA effector complexes and modulate miRNA activity. Nat Cell Biol 11:1143–1149
Gillingham AK, Munro S (2003) Long coiled-coil proteins and membrane traffic. Biochim Biophys Acta 1641:71–85
Gruner R, Santore UJ (1991) Comparative ultrastructural observations of plasmalemmasomes in Pstvd-infected, healthy and artificially stunted tomato leaves. J Phytopathol 131:243–252
Guerra-Guimarães L et al (2015) Proteomic analysis of apoplastic fluid of Coffea arabica leaves highlights novel biomarkers for resistance against Hemileia vastatrix. Front Plant Sci 6:478
Gupta R, Deswal R (2012) Low temperature stress modulated secretome analysis and purification of antifreeze protein from Hippophae rhamnoides, a Himalayan wonder plant. J Proteome Res 11:2684–2696
Hala M et al (2008) An exocyst complex functions in plant cell growth in Arabidopsis and tobacco. Plant Cell 20:1330–1345
He B, Guo W (2009) The exocyst complex in polarized exocytosis. Curr Opin Cell Biol 21:537–542
He B, Xi F, Zhang X, Zhang J, Guo W (2007) Exo70 interacts with phospholipids and mediates the targeting of the exocyst to the plasma membrane. Embo J 26:4053–4065
Heider MR, Munson M (2012) Exorcising the exocyst complex. Traffic 13:898–907
Herman EM, Lamb CJ (1992) Arabinogalactan-rich glycoproteins are localized on the cell surface and in intravacuolar multivesicular bodies. Plant Physiol 98:264–272
Hong W, Lev S (2014) Tethering the assembly of SNARE complexes. Trends Cell Biol 24:35–43
Hsu SC, Hazuka CD, Foletti DL, Scheller RH (1999) Targeting vesicles to specific sites on the plasma membrane: the role of the sec6/8 complex. Trends Cell Biol 9:150–153
Hurley JH (2008) ESCRT complexes and the biogenesis of multivesicular bodies. Curr Opin Cell Biol 20:4–11
Jahn R, Scheller RH (2006) SNAREs—engines for membrane fusion. Nat Rev Mol Cell Biol 7:631–643
Katsiarimpa A, Anzenberger F, Schlager N, Neubert S, Hauser MT, Schwechheimer C, Isono E (2011) The Arabidopsis deubiquitinating enzyme AMSH3 interacts with ESCRT-III subunits and regulates their localization. Plant Cell 23:3026–3040
Kinseth MA, Anjard C, Fuller D, Guizzunti G, Loomis WF, Malhotra V (2007) The Golgi-associated protein GRASP is required for unconventional protein secretion during development. Cell 130:524–534
Klionsky DJ (2007) Autophagy: from phenomenology to molecular understanding in less than a decade. Nat Rev Mol Cell Biol 8:931–937
Kourembanas S (2015) Exosomes: Vehicles of Intercellular Signaling, Biomarkers, and Vectors of Cell Therapy. Annu Rev Physiol 77:13–27
Krause C, Richter S, Knoll C, Jurgens G (2013) Plant secretome—from cellular process to biological activity. Biochim Biophys Acta 1834:2429–2441
Kulich I et al (2013) Arabidopsis exocyst subcomplex containing subunit EXO70B1 is involved in autophagy-related transport to the vacuole. Traffic 14:1155–1165
Kulich I, Vojtikova Z, Glanc M, Ortmannova J, Rasmann S, Zarsky V (2015) Cell wall maturation of Arabidopsis trichomes is dependent on exocyst subunit EXO70H4 and involves callose deposition. Plant Physiol 168:120–131
Langhans M, Forster S, Helmchen G, Robinson DG (2011) Differential effects of the brefeldin A analogue (6R)-hydroxy-BFA in tobacco and Arabidopsis. J Exp Bot 62:2949–2957
Lee MC, Miller EA, Goldberg J, Orci L, Schekman R (2004) Bi-directional protein transport between the ER and Golgi. Annu Rev Cell Dev Biol 20:87–123
Lehtonen MT et al (2014) Protein secretome of moss plants (Physcomitrella patens) with emphasis on changes induced by a fungal elicitor. J Proteome Res 13:447–459
Lin Y et al. (2015) EXPO and Autophagosomes are Distinct Organelles in Plants. Plant physiology. (in press)
Liu J, Zuo X, Yue P, Guo W (2007) Phosphatidylinositol 4,5-bisphosphate mediates the targeting of the exocyst to the plasma membrane for exocytosis in mammalian cells. Mol Biol Cell 18:4483–4492
Lu YJ et al (2012) Patterns of plant subcellular responses to successful oomycete infections reveal differences in host cell reprogramming and endocytic trafficking. Cell Microbiol 14:682–697
Malhotra V (2013) Unconventional protein secretion: an evolving mechanism. Embo J 32:1660–1664
Manjithaya R, Anjard C, Loomis WF, Subramani S (2010) Unconventional secretion of Pichia pastoris Acb1 is dependent on GRASP protein, peroxisomal functions, and autophagosome formation. J Cell Biol 188:537–546
Manjithaya R, Subramani S (2010) Role of autophagy in unconventional protein secretion. Autophagy 6:650–651
Meyer D, Pajonk S, Micali C, O'Connell R, Schulze-Lefert P (2009) Extracellular transport and integration of plant secretory proteins into pathogen-induced cell wall compartments. Plant J 57:986–999
Michaelis S (1993) STE6, the yeast a-factor transporter. Semin Cell Biol 4:17–27
Miyanishi M, Tada K, Koike M, Uchiyama Y, Kitamura T, Nagata S (2007) Identification of Tim4 as a phosphatidylserine receptor. Nature 450:435–439
Mobius W, van Donselaar E, Ohno-Iwashita Y, Shimada Y, Heijnen HFG, Slot JW, Geuze HJ (2003) Recycling compartments and the internal vesicles of multivesicular bodies harbor most of the cholesterol found in the endocytic pathway. Traffic 4:222–231
Mu J et al (2014) Interspecies communication between plant and mouse gut host cells through edible plant derived exosome-like nanoparticles. Mol Nutr Food Res 58:1561–1573
Muller HM, Steringer JP, Wegehingel S, Bleicken S, Munster M, et al (2015) Formation of disulfide bridges drives oligomerization, membrane pore formation, and translocation of fibroblast growth factor 2 to cell surfaces. J Biol Chem 290:8925–8937
Munson M, Novick P (2006) The exocyst defrocked, a framework of rods revealed. Nat Struct Mol Biol 13:577–581
Murthy M, Garza D, Scheller RH, Schwarz TL (2003) Mutations in the exocyst component Sec5 disrupt neuronal membrane traffic, but neurotransmitter release persists. Neuron 37:433–447
Naramoto S et al (2014) Insights into the localization and function of the membrane trafficking regulator GNOM ARF-GEF at the Golgi apparatus in Arabidopsis. Plant Cell 26:3062–3076
Nielsen ME, Feechan A, Bohlenius H, Ueda T, Thordal-Christensen H (2012) Arabidopsis ARF-GTP exchange factor, GNOM, mediates transport required for innate immunity and focal accumulation of syntaxin PEN1. Proc Natl Acad Sci U S A 109:11443–11448
Nielsen ME, Thordal-Christensen H (2013) Transcytosis shuts the door for an unwanted guest. Trends Plant Sci 18:611–616
Nolte-'t Hoen EN, Buschow SI, Anderton SM, Stoorvogel W, Wauben MH (2009) Activated T cells recruit exosomes secreted by dendritic cells via LFA-1. Blood 113:1977–1981
Nosanchuk JD, Nimrichter L, Casadevall A, Rodrigues ML (2008) A role for vesicular transport of macromolecules across cell walls in fungal pathogenesis. Commun Integr Biol 1:37–39
Novick P, Field C, Schekman R (1980) Identification of 23 complementation groups required for post-translational events in the yeast secretory pathway. Cell 21:205–215
Nunes CC, Dean RA (2012) Host-induced gene silencing: a tool for understanding fungal host interaction and for developing novel disease control strategies. Mol Plant Pathol 13:519–529
O'Connell RJ, Panstruga R (2006) Tete a tete inside a plant cell: establishing compatibility between plants and biotrophic fungi and oomycetes. New Phytol 171:699–718
Osborne AR, Rapoport TA, van den Berg B (2005) Protein translocation by the Sec61/SecY channel. Annu Rev Cell Dev Biol 21:529–550
Park M, Jurgens G (2012) Membrane traffic and fusion at post-Golgi compartments. Front Plant Sci 2:111
Pecenkova T et al (2011) The role for the exocyst complex subunits Exo70B2 and Exo70H1 in the plant-pathogen interaction. J Exp Bot 62:2107–2116
Pereira C, Pereira S, Pissarra J (2014) Delivering of proteins to the plant vacuole—an update. Int J Mol Sci 15:7611–7623
Pinedo M, Regente M, Elizalde M, Quiroga IY, Pagnussat LA, Jorrin-Novo J, Maldonado A, de la Canal L (2012) Extracellular sunflower proteins: evidence on non-classical secretion of a jacalin-related lectin. Protein Pept Lett 19:270–276
Poulsen CP, Dilokpimol A, Geshi N (2015) Arabinogalactan biosynthesis: implication of AtGALT29A enzyme activity regulated by phosphorylation and co-localized enzymes for nucleotide sugar metabolism in the compartments outside of the Golgi apparatus. Plant Signal Behav 10:e984524
Poulsen CP, Dilokpimol A, Mouille G, Burow M, Geshi N (2014) Arabinogalactan glycosyltransferases target to a unique subcellular compartment that may function in unconventional secretion in plants. Traffic 15:1219–1234
Prado N, Alche Jde D, Casado-Vela J, Mas S, Villalba M, Rodriguez R, Batanero E (2014) Nanovesicles are secreted during pollen germination and pollen tube growth: a possible role in fertilization. Mol Plant 7:573–577
Rabouille C, Malhotra V, Nickel W (2012) Diversity in unconventional protein secretion. J Cell Sci 125:5251–5255
Raiborg C, Stenmark H (2009) The ESCRT machinery in endosomal sorting of ubiquitinylated membrane proteins. Nature 458:445–452
Raposo G, Nijman HW, Stoorvogel W, Liejendekker R, Harding CV, Melief CJ, Geuze HJ (1996) B lymphocytes secrete antigen-presenting vesicles. J Exp Med 183:1161–1172
Record M, Subra C, Silvente-Poirot S, Poirot M (2011) Exosomes as intercellular signalosomes and pharmacological effectors. Biochem Pharmacol 81:1171–1182
Regente M, Corti-Monzon G, Maldonado AM, Pinedo M, Jorrin J, de la Canal L (2009) Vesicular fractions of sunflower apoplastic fluids are associated with potential exosome marker proteins. Febs Lett 583:3363–3366
Regente M, Pinedo M, Elizalde M, de la Canal L (2012) Apoplastic exosome-like vesicles: a new way of protein secretion in plants? Plant Signal Behav 7:544–546
Rejon JD et al (2013) Proteomics profiling reveals novel proteins and functions of the plant stigma exudate. J Exp Bot 64:5695–5705
Robinson DG, Sieber H, Kammerloher W, Schaffner AR (1996) PIP1 aquaporins are concentrated in plasmalemmasomes of Arabidopsis thaliana mesophyll. Plant Physiol 111:645–649
Rybak K et al (2014) Plant cytokinesis is orchestrated by the sequential action of the TRAPPII and exocyst tethering complexes. Dev Cell 29:607–620
Safavian D, Jamshed M, Sankaranarayanan S, Indriolo E, Samuel MA, Goring DR (2014) High humidity partially rescues the Arabidopsis thaliana exo70A1 stigmatic defect for accepting compatible pollen. Plant Reprod 27:121–127
Sanchez-Wandelmer J, Reggiori F (2013) Amphisomes: out of the autophagosome shadow? Embo J 32:3116–3118
Scheuring D, Viotti C, Kruger F, Kunzl F, Sturm S, Bubeck J, Hillmer S, Frigerio L, Robinson DG, Pimpl P, Schumacher K (2011) Multivesicular bodies mature from the trans-Golgi network/early endosome in Arabidopsis. Plant Cell 23:3463–3481
Schneider A, Simons M (2013) Exosomes: vesicular carriers for intercellular communication in neurodegenerative disorders. Cell Tissue Res 352:33–47
Schoberer J, Liebminger E, Botchway SW, Strasser R, Hawes C (2013) Time-resolved fluorescence imaging reveals differential interactions of N-glycan processing enzymes across the Golgi stack in planta. Plant Physiol 161:1737–1754
Schoberer J, Strasser R (2011) Sub-compartmental organization of Golgi-resident N-glycan processing enzymes in plants. Mol Plant 4:220–228
Schorey JS, Cheng Y, Singh PP, Smith VL (2015) Exosomes and other extracellular vesicles in host-pathogen interactions. EMBO Rep 16:24–43
Simons M, Raposo G (2009) Exosomes—vesicular carriers for intercellular communication. Curr Opin Cell Biol 21:575–581
Spitzer C, Reyes FC, Buono R, Sliwinski MK, Haas TJ, Otegui MS (2009) The ESCRT-related CHMP1A and B proteins mediate multivesicular body sorting of auxin carriers in Arabidopsis and are required for plant development. Plant Cell 21:749–766
Steringer JP et al (2012) Phosphatidylinositol 4,5-bisphosphate (PI(4,5)P2)-dependent oligomerization of fibroblast growth factor 2 (FGF2) triggers the formation of a lipidic membrane pore implicated in unconventional secretion. J Biol Chem 287:27659–27669
Stuffers S, Sem Wegner C, Stenmark H, Brech A (2009) Multivesicular endosome biogenesis in the absence of ESCRTs. Traffic 10:925–937
Takemoto D, Jones DA, Hardham AR (2003) GFP-tagging of cell components reveals the dynamics of subcellular re-organization in response to infection of Arabidopsis by oomycete pathogens. Plant J 33:775–792
Terbush DR, Guo W, Dunkelbarger S, Novick P (2001) Purification and characterization of yeast exocyst complex. Methods Enzymol 329:100–110
TerBush DR, Maurice T, Roth D, Novick P (1996) The exocyst is a multiprotein complex required for exocytosis in Saccharomyces cerevisiae. Embo J 15:6483–6494
Ting AE, Hazuka CD, Hsu SC, Kirk MD, Bean AJ, Scheller RH (1995) rSec6 and rSec8, mammalian homologs of yeast proteins essential for secretion. Proc Natl Acad Sci U S A 92:9613–9617
Torrado LC, Temmerman K, Muller HM, Mayer MP, Seelenmeyer C, Backhaus R, Nickel W (2009) An intrinsic quality-control mechanism ensures unconventional secretion of fibroblast growth factor 2 in a folded conformation. J Cell Sci 122:3322–3329
Trajkovic K et al (2008) Ceramide triggers budding of exosome vesicles into multivesicular endosomes. Science 319:1244–1247
Tzfadia O, Galili G (2013) The Arabidopsis exocyst subcomplex subunits involved in a golgi-independent transport into the vacuole possess consensus autophagy-associated atg8 interacting motifs. Plant Signal Behav 8:e26732
Valadi H, Ekstrom K, Bossios A, Sjostrand M, Lee JJ, Lotvall JO (2007) Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol 9:654–659
Vukasinovic N, Cvrckova F, Elias M, Cole R, Fowler JE, Zarsky V, Synek L (2014) Dissecting a hidden gene duplication: the Arabidopsis thaliana SEC10 locus. PLoS One 9:e94077
Wang F, Shang Y, Fan B, Yu JQ, Chen Z (2014) Arabidopsis LIP5, a positive regulator of multivesicular body biogenesis, is a critical target of pathogen-responsive MAPK cascade in plant basal defense. Plos Pathog 10:e1004243
Wang J et al (2010) EXPO, an exocyst-positive organelle distinct from multivesicular endosomes and autophagosomes, mediates cytosol to cell wall exocytosis in Arabidopsis and tobacco cells. Plant Cell 22:4009–4030
Wideman JG, Leung KF, Field MC, Dacks JB (2014) The cell biology of the endocytic system from an evolutionary perspective. Cold Spring Harb Perspect Biol 6:a016998
Xiang L, Etxeberria E, Van den Ende W (2013) Vacuolar protein sorting mechanisms in plants. Febs J 280:979–993
Yu IM, Hughson FM (2010) Tethering factors as organizers of intracellular vesicular traffic. Annu Rev Cell Dev Biol 26:137–156
Zarsky V, Kulich I, Fendrych M, Pecenkova T (2013) Exocyst complexes multiple functions in plant cells secretory pathways. Curr Opin Plant Biol 16:726–733
Zhang M, Schekman R (2013) Cell biology. Unconventional secretion, unconventional solutions. Science 340:559–561
Zhang X, Orlando K, He B, Xi F, Zhang J, Zajac A, Guo W (2008) Membrane association and functional regulation of Sec3 by phospholipids and Cdc42. J Cell Biol 180:145–158
Zhang Y, Immink R, Liu CM, Emons AM, Ketelaar T (2013) The Arabidopsis exocyst subunit SEC3A is essential for embryo development and accumulates in transient puncta at the plasma membrane. New Phytol 199:74–88
Zhang Y, Liu CM, Emons AM, Ketelaar T (2010) The plant exocyst. J Integr Plant Biol 52:138–146
Zhao T et al (2015) A truncated NLR protein, TIR-NBS2, is required for activated defense responses in the exo70B1 mutant. PLoS Genet 11:e1004945
Zhao Y, Liu J, Yang C, Capraro BR, Baumgart T, Bradley RP, Ramakrishnan N, Xu X, Radhakrishnan R, Svitkina T, Guo W (2013) Exo70 generates membrane curvature for morphogenesis and cell migration. Dev Cell 26:266–278
Acknowledgments
Our research has been supported by grants from the Research Grants Council of Hong Kong (CUHK465112, CUHK466613, CUHK2/CRF/11G, C4011-14R, and AoE/M-05/12), the National Natural Science Foundation of China (31470294), the CAS-Croucher Funding Scheme for Joint Laboratories, and the Shenzhen Peacock Project (KQTD201101).
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling Editor: Peter Nick
Rights and permissions
About this article
Cite this article
Robinson, D.G., Ding, Y. & Jiang, L. Unconventional protein secretion in plants: a critical assessment. Protoplasma 253, 31–43 (2016). https://doi.org/10.1007/s00709-015-0887-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00709-015-0887-1