Abstract
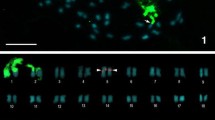
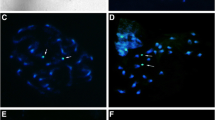
The genus Drimia (syn. Urginea), commonly called squill, represents a species complex, infrageneric delimitation being ill-defined due to morphological variability, population variation within species and polyploidy. In the present study, fluorescent chromosome banding and measurements of nuclear DNA content by flow cytometry were performed in five Indian species of Drimia: Drimia indica, Drimia polyantha, Drimia razii, Drimia wightii and Drimia coromandeliana to elucidate taxonomic relationship and obtain possible insights into the evolutionary processes within this group. All taxa analyzed exhibited similar karyomorphology with subtle differences accounted by nucleolar chromosomes. Nuclear DNA content ranged from 20.41 pg/2C in D. polyantha to 40.80 pg/2C in D. coromandeliana and was positively correlated with chromosome number (r = 0.67, P = 0.02) and total diploid chromatin length (r = 0.59, P = 0.06). Fluorescent chromosome banding revealed the presence of CMA+ve/DAPI−ve signals associated with nucleolar chromosomes presumably coincident with NOR in all species and unique CMA+ve signals in diploid populations of D. indica. Satellite polymorphism between homologous NOR-bearing chromosomes was observed which supports hybrid origin of the taxon. UPGMA dendrogram and scatter diagrams based on karyological parameters indicated a close relationship of D. indica, D. razii and D. polyantha while D. wightii and D. coromandeliana appeared distant. D. wightii appeared more close to D. indica than to all other species based on genome size and karyomorphology. As a whole, D. indica showed high intra-specific variability with populations exhibiting intergrading characters with other species. In conclusion, it is likely that hybridization followed by reproductive isolation of polymorphic forms arising by adaptation to different ecological niches resulted in species diversification of Drimia in India, probably from a common ancestor similar to D. indica.









Similar content being viewed by others
References
Acosta MC, Bernardello G, Guerra M, Moscone EA (2005) Karyotype analysis in several South American species of Solanum and Lycianthes rantonnei (Solanaceae). Taxon 54(3):713–723
Acosta MC, Guerra M, Moscone EA (2012) Karyological relationships among some South American species of Solanum (Solanaceae) based on fluorochrome banding and nuclear DNA amount. Plant Syst Evol 298:1547–1556
Ansari MY (1981) Drimia razii sp. nov. (Liliaceae) from Maharashtra, India. J Bombay Nat Hist Soc 78(3):572–574
Bhowmick BK, Jha TB, Jha S (2012) Chromosome analysis in the dioecious cucurbit Coccinia grandis (L.) Voigt. Chromosom Sci 15:9–15
Blatter E, McCann C (1928) Some new species of plants from Western Ghats. J Bombay Nat Hist Soc 32:733–773
Boraiah G, Fatima TK (1970) Cytotaxonomy of Urginea govindappae sp. nov. Bull Bot Surv India 12:129–131
Deb DB, Dasgupta S (1974) Revision of the genus Urginea Steinheil (Liliaceae) in India. Bull Bot Surv India 16:116–124
Deb DB, Dasgupta S (1981) Fascicles of flora of India 7 Liliaceae-Tribe Scillae. Botanical Survey of India, Howrah
Deb DB, Dasgupta S (1987) On the identity of three new species of Urginea (Liliaceae). J Bombay Nat Hist Soc 84:409–412
Desai N, Kawalkar H, Dixit G (2012) Biosystematics and evolutionary studies in Indian Drimia species. J Syst Evol 50(6):512–518
Dixit GB, Yadav SR (1989) Cytotaxonomical and genetical studies in Urginea Steinh. species from India. Cytologia 54:715–721
Doležel J, Greilhuber J, Suda J (2007) Estimation of nuclear DNA content in plants using flow cytometry. Nat Protoc 2:2233–2244
Fukui K (1996) Plant chromosomes at mitosis. In: Fukui K, Nakayama S (eds) Plant chromosomes: Laboratory methods. CRC press Inc, Boca Raton, pp 1–17
Galbraith DW, Harkins KR, Maddox JM, Ayres NM, Sharma DP, Firoozabady E (1983) Rapid flow cytometric analysis of the cell cycle in intact plant tissues. Science 220(4601):1049–1051
Goldblatt P, Manning JC, Forest F (2012) A review of chromosome cytology in Hyacinthaceae subfamilies Urgineoideae and Hyacinthoideae (tribes Hyacintheae, Massonieae, Pseudoprospereae) in sub-Saharan Africa. S Afr J Bot 83:134–144
Guerra M (2008) Chromosome numbers in plant cytotaxonomy: concepts and implications. Cytogenet Genome Res 120:339–350
Hemadri K, Swahari S (1982) Urginea nagarjunae Hemadri et Swahari-a new species of Liliaceae from India (a new plant discovery). Anc Sci Life 2(2):105–110
Hooker JD (1892) Flora of British India. L. Reeve & Co. Ltd, Kent
Jessop JP (1977) Studies in the bulbous Liliaceae in South Africa 7. The taxonomy of Drimia and certain allied genera. S Afr J Bot 43:265–319
Jha S (1988) Bufadienolides. In: Constabel F, Vasil IK (eds) Cell culture and somatic cell genetics of plants, vol 5. Academic Press, San Diego, pp 179–191
Jha S, Sen S (1980a) A search for scilladienolides in Scilla indica Roxb. Curr Sci 49(7):273–274
Jha S, Sen S (1980b) A natural hexaploid of Urginea indica Kunth (Indian Squill). Cell Chromosome Newslett 3(3):57–58
Jha S, Sen S (1981) Bufadienolides in different chromosomal races of Indian squill. Phytochemistry 20(3):524–526
Jha S, Sen S (1983a) Chromosome study of diploid Indian squill, Urginea indica Kunth. Cytologia 48(1):79–86
Jha S, Sen S (1983b) Chromosome study of polyploid Indian squill, Urginea indica Kunth. Cytologia 48(2):407–418
Jha S, Sen S (1983c) Quantitation of principal bufadienolides in different cytotypes of Urginea indica. Planta Med 47(1):43–45
Jha TB, Yamamoto M (2012) Application of EMA, fluorescence staining and FISH of rDNA in analysis of Aloe vera (L.) Burm. f. chromosomes. Bull Fac Agric Kagoshima Univ 62:83–89
Kron P, Suda J, Husband BC (2007) Applications of flow cytometry to evolutionary and population biology. Annu Rev Ecol Evol Syst 38:847–876
Levan A, Fredga K, Sandberg AA (1964) Nomenclature for centromeric position on chromosomes. Hereditas 52(2):201–220
Linnaeus C (1753) Species Plantarum. Holmiae, Stockholm
Manning JC, Goldblatt P, Fay MF (2004) A revised generic synopsis of Hyacinthaceae in sub-Saharan Africa, based on molecular evidence, including new combinations and the new tribe Pseudoprospereae. Edinb J Bot 60(3):533–568
Moscone EA, Lambrou M, Ehrendorfer F (1996) Fluorescent chromosome banding in the cultivated species of Capsicum (Solanaceae). Plant Syst Evol 202:37–63
Mulholland DA, Schwikkard SL, Crouch NR (2013) The chemistry and biological activity of the Hyacinthaceae. Nat Prod Rep 30(9):1165–1210
Nath S, Saha PS, Jha S (2014a) Medicinal bulbous plants: biology, phytochemistry and biotechnology. In: Ramawat KG, Mérillon JM (eds) Bulbous plants biotechnology. CRC Press Inc, Boca Raton, pp 338–369
Nath S, Mallick SK, Jha S (2014b) An improved method of genome size estimation by flow cytometry in five mucilaginous species of Hyacinthaceae. Cytometry A. doi:10.1002/cyto.a.22489
Paszko B (2006) A critical review and a new proposal of karyotype asymmetry indices. Plant Syst Evol 258:39–48
Pfosser M, Speta F (1999) Phylogenetics of Hyacinthaceae based on plastid DNA sequences. Ann Mo Bot Gard 86(4):852–875
Pikaard CS (1999) Nucleolar dominance and silencing of transcription. Trends Plant Sci 4(2):478–483
Schweizer D (1976) Reverse fluorescent chromosome banding with chromomycin and DAPI. Chromosoma 58(4):307–324
Sokal RR, Rohlf FJ (1995) Biometry: the principles and practice of statistics in biological research. W. H. Freeman & Co., New York
Stebbins GL (1971) Chromosomal changes, genetic recombination and speciation. In: Barrington EJW, Willis AJ (eds) Chromosomal Evolution in Higher Plants. Edward Arnold Publishers. Pvt. Ltd., London, pp 72–123
Stedje B (1987) A revision of the genus Drimia (Hyacinthaceae) in East Africa. Nord J Bot 7(6):655–666
Stedje B (2001) Generic delimitation of Hyacinthaceae, with special emphasis on sub-Saharan genera. Syst Geogr Plants 71:449–454
Steinheil A (1834) Note sur le genre Urginea nouvellement formé dans la famille des Liliacées. Ann Sci Nat Bot 2(1):321–332
Steinheil A (1836) Quelques observations relatives aux genres Scilla et Urginea. Deux genres à établir dans la famille des Liliacées et description d’une spéce nouvelle. Ann Sci Nat Bot 2(6):272–286
Yadav SR, Dixit GB (1990) Cytotaxonomical Studies in Indian Urginea Steinhill Species. Cytologia 55:293–300
Zarco CR (1986) A new method for estimating karyotype asymmetry. Taxon 35(3):526–530
Acknowledgments
SN is grateful to the Department of Science and Technology, GOI for the award of INSPIRE Fellowship. We thank Prof. Jaroslav Doležel, Institute of Experimental Botany, Olomouc, Czech Republic, for providing the seeds for reference standard and Prof. SR Yadav and Dr. Manoj Lekhak, Department of Botany, Shivaji University, Kolhapur, Maharashtra, India, for providing and identifying the plant materials. We also thank CU-BD CoE for Nanobiotechnology, CRNN, University of Calcutta for instrument facilities. Financial assistance from Council of Scientific and Industrial Research (CSIR), Govt. of India to SJ is gratefully acknowledged.
Conflict of interest
We declared here that we have no conflict of interest for the article to be published in the Protoplasma.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling Editor: Peter Nick
Rights and permissions
About this article
Cite this article
Nath, S., Jha, T.B., Mallick, S.K. et al. Karyological relationships in Indian species of Drimia based on fluorescent chromosome banding and nuclear DNA amount. Protoplasma 252, 283–299 (2015). https://doi.org/10.1007/s00709-014-0679-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00709-014-0679-z