Abstract
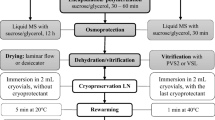
In the present study, polyembryoids of oil palm (Elaeis guineensis Jacq.) were cryopreserved with successful revival of 68 % for the first time using the droplet vitrification technique. Excised polyembryoids (3–5-mm diameter) from 3-month-old in vitro cultures were pre-cultured for 12 h in liquid Murashige and Skoog medium supplemented with 0.5 M sucrose. The polyembryoids were osmoprotected in loading solution [10 % (w/v) dimethyl sulphoxide (DMSO) plus 0.7 M sucrose] for 30 min at room temperature and then placed on aluminium strips where they were individually drenched in chilled droplets of vitrification solution (PVS2) [30 % (w/v) glycerol plus 15 % (w/v) ethylene glycol (EG) plus 15 % (w/v) DMSO plus 0.4 M sucrose] for 10 min. The aluminium strips were enclosed in cryovials which were then plunged quickly into liquid nitrogen and kept there for 1 h. The polyembryoids were then thawed and unloaded (using 1.2 M sucrose solution) with subsequent transfer to regeneration medium and stored in zero irradiance. Following for 10 days of storage, polyembryoids were cultured under 16 h photoperiod of 50 μmol m−2 s−1 photosynthetic photon flux density, at 23 ± 1 °C. Post-thaw growth recovery of 68 % was recorded within 2 weeks of culture, and new shoot development was observed at 4 weeks of growth. Scanning electron microscopy revealed that successful regeneration of cryopreserved polyembryoids was related to maintenance of cellular integrity, presumably through PVS2 exposure for 10 min. The present study demonstrated that cryopreservation by droplet vitrification enhanced the regeneration percentages of oil palm in comparison with the conventional vitrification method previously reported.





Similar content being viewed by others
Abbreviations
- ISSR:
-
Inter-simple sequence repeats
- LN:
-
Liquid nitrogen
- LS:
-
Loading solution
- MC:
-
Moisture content
- MS:
-
Murashige and Skoog
- PPFD:
-
Photosynthetic photon flux density
- PVS2:
-
Plant vitrification solution 2
- RAPD:
-
Random amplified polymorphic DNA
- SEM:
-
Scanning electron microscope
- VS:
-
Vitrification solution
References
Benson EE, Harding K, Smith H (1989) Variation in recovery of cryopreserved shoot-tips of Solanum tuberosum exposed to different pre- and post-freeze light regimes. CryoLetters 10:323–344
Chen XL, Li JH, Xin X, Zhang ZE, Xin PP, Lu XX (2011) Cryopreservation of in vitro-grown apical meristems of Lilium by droplet-vitrification. South African J Bot 77:397–403. doi:10.1016/j.sajb.2010.10.005
Cochard B, Durand-Gasselin T, Amblard P, Konan EK, Gogor S (2000) Performance of adult oil palm clones. In: Emerging technologies and opportunities in the next millennium. Agriculture conference: proceedings of 1999 PORIM international palm oil congress (PIPOC 1999). Kuala Lumpur, Malaysia, pp 53-64
Condello E, Caboni E, Andre E, Piette B, Druart R, Swennen R, Panis B (2011) Cryopreservation of apple in vitro axillary buds using droplet-vitrification. CryoLetters 32:75–85
Corley RHV, Tinker PB (2008) The Oil Palm. Blackwell Science Ltd., Oxford
Coste A, Halmagyi A, Butiuc-Keul AL, Deliu C, Coldea G, Hurdu B (2012) In vitro propagation and cryopreservation of Romanian endemic and rare Hypericum species. Plant Cell Tiss Organ Cult 110:213–226. doi:10.1007/s11240-012-0144-7
Dumet D, Engelmann F, Chabrillange N, Duval Y (1993) Cryopreservation of oil palm (Elaeis guineensis Jacq.) somatic embryos involving a desiccation step. Plant Cell Rep 12(352):355
Duncan DB (1955) Multiple range and multiple F test. Biometrics 11:1–42
Engelmann F (2004) Plant cryopreservation: progress and perspectives. In Vitro Cell Dev Biol Plant 40:427–433. doi:10.1079/IVP2004541
Engelmann F, Engels JMM (2002) Technologies and strategies for ex situ conservation. In: Engels JMM, Ramanatha RV, Brown AHD, Jackson MT (eds) Managing plant genetic diversity. (International Plant Genetic Resources Institute). CABI Publishing, Rome, pp 89–103
Engelmann F, Duval Y, Dereuddre J (1985) Survival and proliferation of oil palm (Elaeis guineensis Jacq.) somatic embryos after freezing in liquid nitrogen. Comptes Rendus de l’Académie des Sciences Paris 301, Sér. III: 111-116
Fábián A, Jäger K, Darkó É, Barnabás B (2008) Cryopreservation of wheat (Triticum aestivum L.) egg cells by vitrification. Acta Physiol Plant 30:737–744. doi:10.1007/s11738-008-0176-0
Gagliardi RF, Pacheco GP, Carneiro LA, Valls JFM, Vieira MLC, Mansur E (2003) Cryopreservation of Arachis species by vitrification of in vitro-grown shoot apices and genetic stability of recovered plants. CryoLetters 24:103–110
Gallard A, Panis B, Dorion N, Swennen R, Grapin A (2008) Cryopreservation of Pelargonium apices by droplet-vitrification. CryoLetters 29:243–251
Gantait S, Sinniah UR (2013) Storability, post-storage conversion and genetic stability assessment of alginate-encapsulated shoot tips of monopodial orchid hybrid Aranda Wan Chark Kuan ‘Blue’ × Vanda coerulea Grifft. ex. Lindl. Plant Biotechnology Rep 7:257–266. doi:10.1007/s11816-012-0257-9
Gantait S, Mandal N, Bhattacharyya S, Das PK (2010) An elite protocol for accelerated quality-cloning in Gerbera jamesonii Bolus cv. Sciella. In Vitro Cellular Dev Biol-Plant 46:537–548. doi:10.1007/s11627-010-9319-2
Gantait S, Sinniah UR, Mandal N, Das PK (2012) Direct induction of protocorm-like bodies from shoot tips, plantlet formation, and clonal fidelity analysis in Anthurium andreanum cv. CanCan. Plant Growth Regul 67:257–270. doi:10.1007/s10725-012-9684-4
Gonzalez-Arnao MT, Panta A, Roca WM, Escobar RH, Engelmann F (2008) Development and large scale application of cryopreservation techniques for shoot and somatic embryo cultures of tropical crops. Plant Cell Tiss Organ Cult 92:1–13. doi:10.1007/s11240-007-9303-7
Guzmán-García E, Bradaї F, Sánchez-Romero C (2013) Cryopreservation of avocado embryogenic cultures using the droplet-vitrification method. Acta Physiol Plant 35:183–193. doi:10.1007/s11738-012-1062-3
Harding K (2004) Genetic integrity of cryopreserved plant cells: a review. CryoLetters 25:3–22
Harding K, Benson EE (2000) Analysis of chloroplast DNA in Solanum tuberosum plantlets derived from cryopreservation. CryoLetters 21:279–288
Harding K, Benson EE (2001) The use of microsatellite analysis in Solanum tuberosum L. in vitro plantlets derived from cryopreserved germplasm. CryoLetters 22:199–208
Harding K, Millam S (2000) Analysis of chromatin, nuclear DNA and organelle composition in somatic hybrids between Solanum tuberosum and Solanum sanctae-rosae. Theor Appl Genet 101:939–947. doi:10.1007/s001220051565
Hazubska-Przbyl T, Chmielarz P, Michalak M, Bojarczuk K (2010) Cryopreservation of embryogenic tissues of Picea omorika (Serbian spruce). Plant Cell Tiss Organ Cult 102:35–44. doi:10.1007/s11240-010-9701-0
Heringer AS, Steinmacher DA, Fraga HPF, Vieira LN, Ree JF, Guerra MP (2013) Global DNA methylation profiles of somatic embryos of peach palm (Bactris gasipaes Kunth) are influenced by cryoprotectants and droplet-vitrification cryopreservation. Plant Cell Tiss Organ Cult 114:365–372. doi:10.1007/s11240-013-0331-1
Hong SR, Yin MH (2009) High-efficiency vitrification protocols for cryopreservation of in vitro grown shoot tips of rare and endangered plant Emmenopterys henryi Oliv. Plant Cell Tiss Organ Cult 99:217–226. doi:10.1007/s11240-009-9598-7
Hong SR, Yin MH, Shao XH, Wang AP, Xu WH (2009) Cryopreservation of embryogenic callus of Dioscorea bulbifera by vitrification. CryoLetters 30:64–75
Huang CN, Wang JH, Yan QS, Zhang ZQ, Yan QF (1995) Plant regeneration from rice (Oryza sativa L.) embryogenic suspension cells cryopreserved by vitrification. Plant Cell Rep 14:730–734. doi:10.1007/BF00232657
Ishikawa K, Harata K, Mili M, Sakai A, Yoshimatsu K, Shimomura K (1997) Cryopreservation of zygotic embryos of Japanese terrestrial orchid (Bletilla striata) by vitrification. Plant Cell Rep 16:754–757. doi:10.1007/s002990050314
Jiwu Z, Ganjun Y, Qiuming Z (2007) Micropropagation and cryopreservation of in vitro shoot tips of ‘Suizhonghong’ papaya. Acta Hortic 760:217–224
Johnston JW, Benson EE, Harding K (2009) Cryopreservation induces temporal DNA methylation epigenetic changes and differential transcriptional activity in Ribes germplasm. Plant Physiol Bioch 4:123–131
Kaity A, Ashmore SE, Drew RA, Dulloo ME (2008) Assessment of genetic and epigenetic changes following cryopreservation in papaya. Plant Cell Rep 27:1529–1539. doi:10.1007/s00299-008-0558-1
Khawniam T, Te-chato S (2012) Cryopreservation of embryogenic callus of hybrid tenera oil palm by dehydration technique and evaluation of somaclonal variation by SSR marker. J Agri Technol 8:2115–2125
Kim HH, Lee YG, Park SU, Lee SC, Baek HJ, Cho EG, Engelmann F (2009) Development of alternative loading solutions in droplet-vitrification procedures. CryoLetters 30:291–299
Kohmura H, Ikeda Y, Sakai A (1994) Cryopreservation of apical meristems of Japanese shallot (Allium wakegi) by vitrification and subsequent high plant regeneration. CryoLetters 15:289–298
Konan EK, Durand-Gasselin T, Koadio YJ, Niamké AC, Dumet D, Duval Y, Rival A, Engelmann F (2007) Field development of oil palms (Elaeis guineensis Jacq.) originating from cryopreserved stabilized polyembryonic cultures (SPCs). CryoLetters 28:377–386
Le Bras C, Le Besnerais PH, Hamama L, Grapin A (2014) Cryopreservation of ex-vitro-grown Rosa chinensis ‘Old Blush’ buds using droplet-vitrification and encapsulation-dehydration. Plant Cell Tiss Organ Cult 116:235–242. doi:10.1007/s11240-013-0400-5
Leunufna S, Keller ERJ (2003) Investigating a new cryopreservation protocol for yams (Dioscorea spp.). Plant Cell Rep 21:1159–1166. doi:10.1007/s00299-003-0652-3
Li MJ, Zhao XT, Hong SR, Zhang XL, Li P, Liu J, Xie CH (2009) Cryopreservation of plantlet nodes of Dioscorea opposita Thunb. using vitrification method. CryoLetters 30:19–28
Martinez-Montero ME, Martinez J, Engelmann F (2008) Cryopreservation of sugarcane somatic embryos. CryoLetters 29:229–242
Matsumoto T, Sakai A, Yamada K (1994) Cryopreservation of in vitro-grown apical meristems of wasabi (Wasabia japonica) by vitrification and subsequent high plant regeneration. Plant Cell Rep 13:442–446. doi:10.1007/BF00231963
Mielke S (1991) Economic prospects for oilseeds, oils and fats toward the 21st century. In: Basiron Y and Ibrahim A (eds) The proceedings of the 1991 PORIM International Palm Oil Conference. Module IV: promotion and marketing. September 9–14, Palm Oil Research Institute of Malaysia, Kuala Lumpur, pp 1-16
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15:473–479. doi:10.1111/j.1399-3054.1962.tb08052.x
Niino T, Sakai A, Yakuwa H, Nojiri K (1992) Cryopreservation of in vitro-grown shoot tips of apple and pear by vitrification. Plant Cell Tiss Organ Cult 28:261–266. doi:10.1007/BF00036122
Palanyandy SR, Suranthran P, Gantait S, Sinniah UR, Subramaniam S, Aziz MA, Sarifa SRSA, Roowi SH (2013) In vitro developmental study of oil palm (Elaeis guineensis Jacq.) polyembryoids from cell suspension using scanning electron microscopy. Acta Physiol Plant 35:1727–1733. doi:10.1007/s11738-012-1201-x
Panis B, Piette B, Swennen R (2005) Droplet vitrification of apical meristems: a cryopreservation protocol applicable to all Musaceae. Plant Sci 168:45–55. doi:10.1016/j.plantsci.2004.07.022
Pawłowska B, Szewczyk-Taranek B (2014) Droplet vitrification cryopreservation of Rosa canina and Rosa rubiginosa using shoot tips from in situ plants. Sci Hort 168:151–156. doi:10.1016/j.scienta.2013.12.016
Razaq M, Heikrujam M, Chetri SK, Agrawal V (2013) In vitro clonal propagation and genetic fidelity of the regenerants of Spilanthes calva DC. using RAPD and ISSR marker. Physiol Mol Biol Plants 19:251–260. doi:10.1007/s12298-012-0152-4
Reinhoud PJ, Schrijnemakers EWM, Iren F, Kijne JW (1995) Vitrification and a heat-shock treatment improve cryopreservation of tobacco cell suspension compared to two-step freezing. Plant Cell Tiss Organ Cult 42:261–267. doi:10.1007/BF00029997
Ryynänen L, Aronen T (2005) Genome fidelity during short- and long-term tissue culture and differentially cryostored meristems of silver birch (Betula pendula). Plant Cell Tiss Organ Cult 83:21–32. doi:10.1007/s11240-005-3396-7
Sakai A, Engelmann F (2007) Vitrification, encapsulation-vitrification and droplet-vitrification: a review. CryoLetters 28:151–172
Sakai A, Kobayashi S, Oiyama I (1990) Cryopreservation of nucellar cells of navel orange (Citrus sinensis Osb. var. brasiliensis Tanaka) by vitrification. Plant Cell Rep 9:30–33. doi:10.1007/BF00232130
Sant R, Panis B, Taylor M, Tyagi A (2008) Cryopreservation of shoot-tips by droplet vitrification applicable to all taro (Colocasia esculenta var. esculenta) accessions. Plant Cell Tiss Organ Cult 92:107–111. doi:10.1007/s11240-007-9302-8
Schäfer-Menuhr A, Schumacher HM, Mix-Wagner G (1997) Cryopreservation of potato cultivars: design of a method for routine application in genebanks. Acta Hortic 447:477–482
Senula A, Keller JER, Sanduijav T, Yohannes T (2007) Cryopreservation of cold-acclimated mint (Mentha spp.) shoot tips using a simple vitrification protocol. CryoLetters 28:1–12
Sopalun K, Kanchit K, Ishikawa K (2010) Vitrification-based cryopreservation of Grammatophyllum speciosum protocorm. CryoLetters 31:347–357
Suranthran P, Gantait S, Sinniah UR, Subramaniam S, Sarifa SRSA, Roowi SH (2012) Effect of loading and vitrification solutions on survival of cryopreserved oil palm polyembryoids. Plant Growth Regul 66:101–109. doi:10.1007/s10725-011-9633-7
Thormann I, Dulloo ME, Engels JMM (2006) Techniques of ex situ plant conservation. In: Henry R (ed) Plant Conservation genetics. Centre for Plant Conservation Genetics, Southern Cross University. The Haworth Press inc, Lismore, pp 7–36
Tokatli OY, Akdemir H (2010) Cryopreservation of fraser Photinia (Photinia x fraseri Dress.) via vitrification-based one-step freezing techniques. CryoLetters 31:40–49
Turner S, Krauss SL, Bunn E, Senaratna T, Dixon K, Tan B, Touchell D (2001) Genetic fidelity and viability of Anigozanthos viridis following tissue culture, cold storage and cryopreservation. Plant Sci 161:1099–1106. doi:10.1016/S0168-9452(01)00519-2
Yin MH, Hong SR (2009) Cryopreservation of Dendrobium candidum Wall. ex Lindl. protocorm-like bodies by encapsulation-vitrification. Plant Cell Tiss Organ Cult 98:179–185. doi:10.1007/s11240-009-9550-x
Acknowledgments
The authors are grateful to Yayasan Felda, Malaysia, for the financial support through research grant, Dr. Sharifah SRSA and Dr. Siti Habsah from Felda Biotechnology Centre for the supply of cell suspension culture and Dr. Sanjoy Banerjee (Institute of Bioscience, Universiti Putra Malaysia) along with Dr. Kuttichanthran Subramaniam (Faculty of Veterinary Medicine, Universiti Putra Malaysia) for their assistance during genetic integrity study, and last but not the least, the Department of Crop Science, Faculty of Agriculture, Universiti Putra Malaysia for the research facilities.
Conflict of interest
We, the authors of this article, declare that there is no conflict of interest, and we do not have any financial gain from it.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling Editor: Peter Nick
S. Gantait and U.R. Sinniah conceived the idea and designed the experiments. S. Gantait P. Suranthran and S.R. Palanyandy executed the experiments. S. Gantait, U.R. Sinniah and S. Subramaniam wrote the manuscript.
Rights and permissions
About this article
Cite this article
Gantait, S., Sinniah, U.R., Suranthran, P. et al. Improved cryopreservation of oil palm (Elaeis guineensis Jacq.) polyembryoids using droplet vitrification approach and assessment of genetic fidelity. Protoplasma 252, 89–101 (2015). https://doi.org/10.1007/s00709-014-0660-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00709-014-0660-x