Abstract
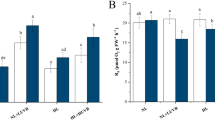
We studied the temporal generation of reactive oxygen species (ROS) in the cyanobacterium Anabaena variabilis PCC 7937 under simulated solar radiation using WG 280, WG 295, WG 305, WG 320, WG 335, WG 345, and GG 400 nm cut-off filters to find out the minimum exposure time and most effective region of the solar spectrum inducing highest level of ROS. There was no significant generation of ROS in all treatments in comparison to the samples kept in the dark during the first 8 h of exposure; however, after 12 h of exposure, ROS were significantly generated in samples covered with 305, 295, or 280 nm cut-off filters. In contrast with ROS, the fragmentation of filaments was predominantly seen in 280 nm cut-off filter covered samples after 12 h of exposure. After 24 h of exposure, ROS levels were significantly higher in all samples than in the dark; however, the ROS signals were more pronounced in 320, 305, 295, or 280 nm cut-off filter covered samples. In contrast, the length of filaments was reduced in 305, 295, or 280 nm cut-off filter covered samples after 24 h of exposure. Thus, fragmentation of the filament was induced by all wavelengths of the UV-B region contrary to the UV-A region where only shorter wavelengths were able to induce the fragmentation. In contrast, ROS were generated by all wavelengths of the solar spectrum after 24 h of exposure; however, shorter wavelengths of both the UV-A and the UV-B regions were more effective in generating ROS in comparison to their higher wavelengths and photosynthetic active radiation (PAR). Moreover, lower wavelengths of UV-B were more efficient than the lower wavelengths of the UV-A radiation. Findings from this study suggest that certain threshold levels of ROS are required to induce the fragmentation of filaments.



Similar content being viewed by others
References
Cockell CS, Rettberg P, Rabbow E, Olsson-Francis K (2011) Exposure of phototrophs to 548 days in low Earth orbit: Microbial selection pressures in outer space and on early earth. ISME J 5:1671–1682
de Almeida SL, Schmidt ÉC, Pereira DT, Kreusch M et al (2013) Effect of ultraviolet-B radiation in laboratory on morphological and ultrastructural characteristics and physiological parameters of selected cultivar of Oryza sativa L. Protoplasma 250:1303–1313
Fischer WF (2008) Life before the rise of oxygen. Nature 455:1051–1052
Foreman J, Demidchik V, Bothwell JHF, Mylona P, Miedema H, Torresk MA, Linstead P, Costa S, Brownlee C, Jonesk JDG, Davies JM, Dolan L (2003) Reactive oxygen species produced by NADPH oxidase regulate plant cell growth. Nature 422:442–446
Foyer CH, Lelandais M, Kunert KJ (1994) Photooxidative stress in plants. Physiol Plant 92:696–717
Gutu A, Kehoe DM (2012) Emerging perspectives on the mechanisms, regulation, and distribution of light color acclimation in cyanobacteria. Mol Plant 5:1–13
Häder D-P (2011) Does enhanced solar UV-B radiation affect marine primary producers in their natural habitats? Photochem Photobiol 87:263–266
Häder D-P, Helbling EW, Williamson CE, Worrest RC (2011) Effects of UV radiation on aquatic ecosystems and interactions with climate change. Photochem Photobiol Sci 10:242–260
Halliwell B, Gutteridge J (2007) Free radicals in biology and medicine, 4th edn. Oxford University Press, Oxford
Hargreaves A, Taiwo FA, Duggan O, Kirk SH, Ahmad SI (2007) Near-ultraviolet photolysis of b-phenylpyruvic acid generates free radicals and results in DNA damage. J Photochem Photobiol B Biol 89:110–116
He Y-Y, Häder D-P (2002) UV-B-induced formation of reactive oxygen species and oxidative damage of the cyanobacterium Anabaena sp: Protective effects of ascorbic acid and n-acetyl-l-cysteine. J Photochem Photobiol B Biol 66:115–124
Karentz D, Cleaver JE, Mitchell DL (1991) DNA damage in the Antarctic. Nature 350:28
Kehoe DM (2010) Chromatic adaptation and the evolution of light color sensing in cyanobacteria. Proc Natl Acad Sci U S A 107:9029–9030
Keshari M, Richa, Sinha RP (2011) Antioxidants as natural arsenal against multiple stresses in cyanobacteria. Int J Pharm Bio Sci 2:B168–B187
Lesser MP (2011) Oxidative stress in tropical marine ecosystems. In: Abele D, Vázquez-Medina JP, Zenteno-Savín T (eds) Oxidative stress in aquatic ecosystems. Blackwell Publishing Ltd, Oxford, pp 9–19
Ma Z, Gao K (2010) Spiral breakage and photoinhibition of Arthrospira platensis (Cyanophyta) caused by accumulation of reactive oxygen species under solar radiation. Environ Exp Bot 68:208–213
Manney GL, Santee ML, Rex M et al (2011) Unprecedented Arctic ozone loss in 2011. Nature 478:469–475
Parmar A, Singh NK, Pandey A, Gnansounou E, Madamwar D (2011) Cyanobacteria and microalgae: a positive prospect for biofuels. Bioresour Technol 102:10163–10172
Pospíšil P (2009) Production of reactive oxygen species by photosystem II. Biochim Biophys Acta Bioenerg 1787:1151–1160
Rastogi RP, Sinha RP (2009) Biotechnological and industrial significance of cyanobacterial secondary metabolites. Biotechnol Adv 27:521–539
Rastogi RP, Singh SP, Häder D-P, Sinha RP (2010) Detection of reactive oxygen species (ROS) by the oxidant-sensing probe 2′,7′-dichlorodihydrofluorescein diacetate in the cyanobacterium Anabaena variabilis PCC 7937. Biochem Biophys Res Commun 397:603–607
Rastogi RP, Singh SP, Häder D-P, Sinha RP (2011) Ultraviolet-B-induced DNA damage and photo repair in the cyanobacterium Anabaena variabilis PCC 7937. Environ Exp Bot 74:280–288
Rastogi RP, Kumari S, Richa, Han T, Sinha RP (2012) Molecular characterization of hot spring cyanobacteria and evaluation of their photoprotective compounds. Can J Microbiol 58:719–727
Richa, Rastogi RP, Kumari S, Singh KL, Kannaujiya VK, Singh G, Kesheri M, Sinha RP (2011) Biotechnological potential of mycosporine-like amino acids and phycobiliproteins of cyanobacterial origin. Biotechnol Bioinforma Bioeng 1:159–171
Rinalducci S, Pedersen JZ, Zolla L (2008) Generation of reactive oxygen species upon strong visible light irradiation of isolated phycobilisomes from Synechocystis PCC 6803. Biochim Biophys Acta Bioenerg 1777:417–424
Safferman RS, Morris ME (1964) Growth characteristics of the blue-green algal virus LPP1. J Bacteriol 88:771–775
Sicora CI, Appleton SE, Brown CM, Chung J, Chandler J, Cockshutt AM, Vass I, Campbell DA (2006) Cyanobacterial psbA families in Anabaena and Synechocystis encode trace, constitutive and UVB-induced D1 isoforms. Biochim Biophys Acta Bioenerg 1757:47–56
Singh SP, Montgomery BL (2011) Determining cell shape: Adaptive regulation of cyanobacterial cellular differentiation and morphology. Trends Microbiol 19:278–285
Singh SP, Häder D-P, Sinha RP (2010) Cyanobacteria and ultraviolet radiation (UVR) stress: Mitigation strategies. Ageing Res Rev 9:79–90
Singh G, Babele PK, Sinha RP, Tyagi MB, Kumar A (2013) Enzymatic and non-enzymatic defense mechanisms against ultraviolet-B radiation in two Anabaena species. Process Biochem 48:796–802
Stanier RY, Cohen-Bazire G (1977) Phototrophic prokaryotes: the cyanobacteria. Annu Rev Microbiol 31:225–274
Van Breusegem F, Dat JF (2006) Reactive oxygen species in plant cell death. Plant Physiol 141:384–390
Vincent WF, Neale PJ (2000) Mechanisms of UV damage to aquatic organisms. In: de Mora SJ, Demers S, Vernet M (eds) The effects of UV radiation on marine ecosystems. Cambridge Univ. Press, Cambridge, pp 149–176
Xie Z, Wang Y, Liu Y, Liu Y (2009) Ultraviolet-B exposure induces photo-oxidative damage and subsequent repair strategies in a desert cyanobacterium Microcoleus vaginatus Gom. Eur J Soil Biol 45:377–382
Yadav S, Sinha RP, Tyagi MB, Kumar A (2011) Cyanobacterial secondary metabolites. Int J Pharma Bio Sci 2:B144–B167
Zehr JP (2011) Nitrogen fixation by marine cyanobacteria. Trends Microbiol 19:162–173
Acknowledgments
R.P. Rastogi is thankful to University Grants Commission, New Delhi, India, for financial support in the form of a fellowship. We are also thankful to Dr. Peter Richter, Friedrich-Alexander Universität, Erlangen-Nürnberg, Germany, for his help during the microscopic analysis.
Conflict of interest
The authors declare no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling Editor: Néstor Carrillo
Shailendra P. Singh and Rajesh P. Rastogi contributed equally to the MS.
Rights and permissions
About this article
Cite this article
Singh, S.P., Rastogi, R.P., Häder, DP. et al. Temporal dynamics of ROS biogenesis under simulated solar radiation in the cyanobacterium Anabaena variabilis PCC 7937. Protoplasma 251, 1223–1230 (2014). https://doi.org/10.1007/s00709-014-0630-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00709-014-0630-3