Abstract
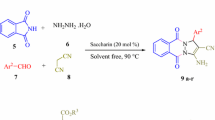
An efficient synthesis of quinazolinone sulfonamide derivatives through four-component reaction is reported. The four-component reaction is based on the reaction of isatoic anhydride, hydrazine hydrate, saccharin, and aromatic aldehydes in the presence of nano-catalyst SBA-Pr-SO3H. Saccharin was used as a novel source of sulfonamide moiety in this reaction.
Graphical abstract








Similar content being viewed by others
References
Ugi I, Dömling A, Hörl W (1994) Endeavour 18:115
Armstrong RM, Combs AP, Tempest PA, Brown SD, Keating TA (1996) Acc Chem Res 29:123
Bienaymé H, Hulme C, Oddon G, Schmitt P (2000) Chem Eur J 6:3321
Harmer MA (2002) In: Clark JH, Macquarrie DJ (eds) Handbook of green chemistry & technology. Blackwell, Oxford
Clark JH (2002) Acc Chem Res 35:791
Wilson K, Adams DJ, Rothenberg G, Clark JH (2000) J Mol Catal A Chem 159:309
Wight AP, Davis ME (2002) Chem Rev 102:3589
Lim MH, Blanford CF, Stein A (1998) Chem Mater 10:467
Fathi Vavsari V, Mohammadi Ziarani G, Badiei A (2015) RSC Adv 5:91686
Karimi B, Vafaeezadeh M (2012) Chem Commun 48:3327
VanGrieken R, Melero JA, Morales G (2005) Appl Catal A 289:143
Fathi Vavsari V, Mohammadi Ziarani G, Badiei A, Balalaie S (2016) J Iran Chem Soc 13:1037
Xue S, McKenna J, Shieh WC, Repic O (2004) J Org Chem 69:6474
Honkanen E, Pippuri A, Kairisalo P, Nore P, Karppanen H, Paakkari I (1983) J Med Chem 26:1433
Zhou Y, Murphy DE, Sun Z, Gregor VE (2004) Tetrahedron Lett 45:8049
Panneerselvam P, Pradeepchandran RV, Sridhar SK (2003) Ind J Pharm Sci 65:268
Refaie FM, Esmat AY, Gawad SMA, Ibrahim AM, Mohamed MA (2005) Lipids Health Dis 4:1
Habib NS, Ismail KA, El-Tombary AA, Abdel AT (2000) Pharmazie 55:495
Jessy EM, Sambanthan AT, Alex J, Sridevi CH, Srinivasan KK (2007) Ind J Pharm Sci 69:476
Alagarsamy V, Solomon VR, Dhanabal K (2007) Bioorg Med Chem 15:235
Georgey H, Abdel-Gawad N, Abbas S (2008) Molecules 13:2557
Guan LP, Jin QH, Tian GR, Chai KY, Quan ZS (2007) J Pharm Pharm Sci 10:254
El-Azab AS, Abdel-Hamide SG, Sayed-Ahmed MM, Hassan GS, El-Hadiyah TM, Al-Shabanah OA, Al-Deeb OA, El-Subbagh HI (2013) Med Chem Res 22:2815
Malawska B (2005) Curr Top Med Chem 5:69
Srivastava VK, Kumar A (2004) Bioorg Med Chem 12:1257
Li J, Meng Y, Liu Y, Feng ZQ, Chen XG (2010) Invest New Drugs 28:132
Chandrika PM, Yakaiah T, Rao ARR, Narsaiah B, Reddy NC, Sridhar V, Rao JV (2008) Eur J Med Chem 43:846
Foote KM, Mortlock AA, Heron NM, Jung FH, Hill GB, Pasquet G, Brady MC, Green S, Heaton SP, Kearney S, Keen NJ (2008) Bioorg Med Chem Lett 18:1904
Cao SL, Feng YP, Jiang YY, Liu SY, Ding GY, Li RT (2005) Bioorg Med Chem Lett 15:1915
Al-Omary FA, Abou-Zeid LA, Nagi MN, Habib ESE, Alaa AM, El-Azab AS, Abdel-Hamide SG, Al-Omar MA, Al-Obaid AM, El-Subbagh HI (2010) Bioorg Med Chem 18:2849
Kołazzek A, Fusiarz I, Ławeccka J, Branowska D (2014) Chemik 68:620
Kataoka T, Iwama T, Setta T, Takagi A (1998) Synthesis 4:423
Deng X, Mani N (2006) Green Chem 8:835
Shi F, Kin Tse M, Zhou S, Pohl MM, Radnik J, Huebner S, Jaehnlisch K, Brueckner A, Beller M (2009) J Am Chem Soc 131:1775
Massah AR, Sayadi S, Ebrahimi S (2012) RSC Adv 2:6606
Bahrami K, Khodaei M, Soheilizad M (2009) J Org Chem 74:9287
Masereel B, Rolin S, Francesco Abbate F, Scozzafava A, Supuran CT (2002) J Med Chem 45:312
Reddy NS, Reddy MM, Cosenza S, Gumireddy K (2004) Bioorg Med Chem Lett 14:4093
Li XH, Yang XL, Ling Y, Fan ZJ, Liang XM, Wang DQ, Chen FH, Li ZM (2005) J Agric Food Chem 53:2202
Hu L, Li ZR, Li Y, Qu J, Ling YH, Jiang JD, Boykin DW (2006) J Med Chem 49:6273
Accinelli C, Koskinen WC, Becker JM, Sadowsky MJ (2007) J Agric Food Chem 55:2677
Ghorab MM, Ismail ZH, Radwan AA, Abdalla M (2013) Acta Pharm 63:1
Abbate F, Casini A, Owa T, Scozzafava A, Supuran CT (2004) Bioorg Med Chem Lett 14:217
Hayun, Yanuar A, Hanafi M, Hudiyono PWS (2011) Bioinformation 7:246
Vanparia SF, Patel TS, Dixit RB, Dixit BC (2013) Med Chem Res 22:5184
Maarouf AR, El-Bendary ER, Goda FE (2004) Arch Pharm Pharm Med Chem 337:527
Ghorab MM, Alsaid MS, Al-Dosari MS, El-Gazzar MG, Parvez MK (2016) Molecules 21:189
Al-Rashood ST, Aboldahab IA, Nagi MN, Abouzeid LA, Abdel-Aziz AAM, Abdel-hamide SG, Youssef KM, Al-Obaida AM, El-Subbagh HI (2006) Bioorg Med Chem 14:8608
Zhou Y, Murphy DE, Sun Z, Gregor VE (2004) Tetrahedron Lett 45:8049
Smirnov GA, Sizova EP, Lukyanov OA, Fedyanin IV, Antipin MY (2003) Russ Chem Bull 52:2444
Ding QS, Zhang JX, Liu MC, Ding JC, Wu HY (2012) J Heterocycl Chem 49:375
Ibrahim SM, Baraka MM, El-Sabbagh OI, Kothayer H (2013) Med Chem Res 22:1488
Agar DJ, Pantaleone DP, Henderson SA, Katritzky AR, Prakash I, Walters DE (1998) Angew Chem Int Ed 37:1802
Balalaie S, Bijanzadeh HR, Mehrparvar S, Rominger F (2016) Synlett 27:782
Balalaie S, Ramezanpour S (2016) Helv Chim Acta 99:138
Fathi V, Ramezanpour S, Balalaie S, Rominger F, Bijanzadeh H (2014) Helv Chim Acta 97:1630
Nikbakht A, Ramezanpour S, Balalaie S, Rominger F (2015) Tetrahedron 71:6790
Alavijeh NS, Zadmard R, Ramezanpour S, Balalaie S, Alavijeh MS, Rominger F (2015) New J Chem 39:6578
Maghari S, Ramezanpour S, Balalaie S, Darvish F, Rominger F, Bijanzadeh HR (2013) J Org Chem 78:6450
Ahadi S, Naghdiani Z, Balalaie S, Rominger F (2015) Tetrahedron 71:6860
Tajbakhsh M, Ramezanpour S, Balalaie S, Bijanzadeh HR (2015) J Heterocycl Chem 52:1559
Mehrparvar S, Balalaie S, Rabbanizadeh M, Rominger F, Ghabraie E (2014) Org Biomol Chem 12:5757
Ghabraie E, Balalaie S, Mehrparvar S, Rominger F (2014) J Org Chem 79:7926
Fathi Vavsari V, Dianati V, Ramezanpour S, Balalaie S (2015) Synlett 26:1955
Sheldrick GM (2012) Bruker analytical X-ray division. Madison, Wisconsin
Sheldrick GM (2015) Acta Cryst C71:3
Acknowledgements
We gratefully acknowledge the Iran National Science Foundation (INSF) for the financial support.
Author information
Authors and Affiliations
Corresponding author
Additional information
Dedicated to Prof. Ali Pourjavadi on the occasion of his 65th birthday.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Balalaie, S., Hekmat, S., Ramezanpour, S. et al. An environmentally friendly approach for the synthesis of quinazolinone sulfonamide. Monatsh Chem 148, 1453–1461 (2017). https://doi.org/10.1007/s00706-017-1924-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00706-017-1924-x