Abstract
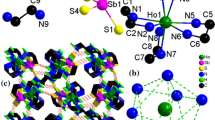
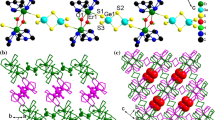
New lanthanide(III) compounds [[Ln(tepa)(Cl)]-[Ln(tepa)(OH)]2(SbSe4)2] n (Ln=Sm, Eu), [H2tepa][[Ln(tepa)(SbSe4)]2(OH)2] (Ln=Eu, Gd, Ho) (tepa=tetraethylenepentamine) were prepared by solvothermal methods. Acting as a bidentate μ-1κ:2κ-SbSe4 bridging ligand, the [SbSe4]3− unit interconnects [[Ln(tepa)]2(OH)2]4+ and [Ln(tepa)Cl]2+ (Ln=Sm, Eu) ions to form one-dimensional coordination polymers [[Ln(tepa)(Cl)][Ln(tepa)(OH)]2(SbSe4)2] n . The [SbSe4]3− unit acts as monodentate ligand to Ln(III) centers in [H2tepa][[Ln(tepa)(SbSe4)]2(OH)2]. The different coordination modes of the [SbSe4]3− units in [[Ln(tepa)(Cl)][Ln(tepa)(OH)]2(SbSe4)2] n and [H2tepa][[Ln(tepa)(SbSe4)]2(OH)2] are attributed to the size of Ln3+ ions. The bidentate μ-1κ:2κ-SbSe4 bridging ligand in [[Ln(tepa)(Cl)][Ln(tepa)(OH)]2(SbSe4)2] n is observed in the lanthanide complexes of tetraselenidoantimonate ligands for the first time. All compounds exhibit steep band gaps between 2.04 and 2.31 eV at room temperature.
Graphical abstract







Similar content being viewed by others
References
Wachhold M, Kanatzidis MG (1999) Inorg Chem 38:3863
Wachhold M, Kanatzidis MG (2000) Inorg Chem 39:2337
Bera TK, Jang JI, Song J-H, Malliakas CD, Freeman AJ, Ketterson JB, Kanatzidis MG (2010) J Am Chem Soc 132:3484
Xiong WW, Athresh EU, Ng YT, Ding JF, Wu T, Zhang QC (2013) J Am Chem Soc 135:1256
Seidlhofer B, Spetzler V, Näther C, Bensch W (2012) J Solid State Chem 187:269
Schaefer M, Näther C, Lehnert N, Bensch W (2004) Inorg Chem 43:2914
Kiebach R, Pienack N, Ordolff ME, Studt F, Bensch W (2006) Chem Mater 18:1196
Liu GN, Guo GC, Chen F, Wang SH, Sun J, Huang JS (2012) Inorg Chem 51:472
Sheldrick WS, Wachhold M (1998) Coord Chem Rev 176:211
Li J, Chen Z, Wang RJ, Proserpio DM (1999) Coord Chem Rev 190–192:707
Sheldrick WS (2000) J Chem Soc Dalton Trans 3041
Seidlhofer B, Pienack N, Bensch W (2010) Z Naturforsch 65b:937
Zhou J, Dai J, Bian GQ, Li CY (2009) Coord Chem Rev 253:1221
Stephan HO, Kanatzidis MG (1996) J Am Chem Soc 118:12226
Vaqueiro P, Chippindale AM, Powell AV (2004) Inorg Chem 43:7963
Lees RJE, Powell AV, Chippindale AM (2005) Polyhedron 24:1941
Bensch W, Näther C, Stähler R (2001) Chem Commun 5:477
Stähler R, Bensch W (2002) Z Anorg Allg Chem 628:1657
Stähler R, Mosel BD, Eckert H, Bensch W (2002) Angew Chem Int Ed 41:4487
Yue CY, Lei XW, Ma YX, Sheng N, Yang YD, Liu GD, Zhai XR (2014) Cryst Growth Des 14:101
Stähler R, Näther C, Bensch W (2003) J Solid State Chem 174:264
Jin QY, Zhu AM, Pan YL, Jia DX, Zhang Y, Gu JS (2009) Z Anorg Allg Chem 635:139
Jia DX, Zhang Y, Zhao QX, Deng J (2006) Inorg Chem 45:9812
Schur M, Rijnberk H, Näther C, Bensch W (1999) Polyhedron 18:101
Schur M, Bensch W (2002) Z Naturforsch 57b:1
Engelke L, Stähler R, Schur M, Näther C, Bensch W, Pöttgen R, Möller MH (2004) Z Naturforsch 59b:869
Bensch W, Näther C, Schur M (1997) Chem Commun 18:1773
Almsick TV, Sheldrick WS (2006) Z Anorg Allg Chem 632:1413
Yue CY, Lei XW, Zang HP, Zhai XR, Feng LJ, Zhao ZF, Zhao JQ, Liu XY (2014) CrystEngComm 16:3424
Möller K, Näther C, Bannwarth A, Bensch W (2007) Z Anorg Allg Chem 633:2635
Stähler R, Bensch W (2001) Eur J Inorg Chem 3073
Schaefer M, Kurowski D, Pfitzner A, Näther C, Rejai Z, Möller K, Ziegler N, Bensch W (2006) Inorg Chem 45:3726
Schaefer M, Stähler R, Kiebach WR, Näther C, Bensch W (2004) Z Anorg Allg Chem 630:1816
Schaefer M, Näther C, Bensch W (2004) Monatsh Chem 135:461
Stähler R, Bensch W (2001) J Chem Soc Dalton Trans 2518
Schaefer M, Engelke L, Bensch W (2003) Z Anorg Allg Chem 629:1912
Lichte J, Lühmann H, Näther C, Bensch W (2009) Z Anorg Allg Chem 635:2021
Nie L, Xiong WW, Li PZ, Han JY, Zhang GD, Yin SM, Zhao YL, Xu R, Zhang QC (2014) J Solid State Chem 220:118
Pan YL, Chen JF, Wang J, Zhang Y, Jia DX (2010) Inorg Chem Commun 13:1569
Tang WW, Chen RH, Zhao J, Jiang WQ, Zhang Y, Jia DX (2012) CrystEngComm 14:5021
Jia DX, Jin QY, Chen JF, Pan YL, Zhang Y (2009) Inorg Chem 48:8286
Chen RH, Tang WW, Jiang WQ, Zhang Y, Jia DX (2013) J Coord Chem 66:650
Jia DX, Zhu AM, Jin QY, Zhang Y, Jiang WQ (2008) J Solid State Chem 181:2370
Zhao J, Liang JJ, Pan YL, Zhang Y, Jia DX (2011) J Solid State Chem 184:1451
Cassol A, Bernardo PDi, Portanova R, Tolazzi M, Tomat G, Zanonato P (1992) J Chem Soc Dalton Trans 469
Zhou J, An LT, Hu FL, Liu X, Zhao RQ, Lin JW (2012) CrystEngComm 14:5544
Zhou J, Hu FL, An LT, Liu X, Meng CY (2012) Dalton Trans 41:11760
Xiao HP, Zhou J, Zhao RQ, Zhang WB, Huang Y (2015) Dalton Trans 44:6032
Liu Y, Tang CY, Han JY, Shen YL, Lu JL, Jia DX (2015) Inorg Chem Commun 60:103
Jin QY, Chen JF, Pan YL, Zhang Y, Jia DX (2010) J Coord Chem 63:1492
Wendlandt WW, Hecht HG (1966) Reflectance spectroscopy. Interscience Publishers, New York
Chen Z, Wang RJ, Dilks KJ, Li J (1999) J Solid State Chem 147:132
Chen Z, Dilks RE, Wang RJ, Lu JY, Li J (1998) Chem Mater 10:3184
Sheldrick GM (1997) SHELXS-97 program for solution of crystal structures. University of Göttingen, Germany
Sheldrick GM (1997) SHELXL-97 program for refinement of crystal structures. University of Göttingen, Germany
Acknowledgments
This work was supported by the National Natural Science Foundation of China (Grant No. 21171123), and the project funded by the Priority Academic Program Development (PAPD) of Jiangsu Higher Education Institutions.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Sun, P., Liu, S., Han, J. et al. Solvothermal syntheses, crystal structures, and properties of new lanthanide compounds based on tetraselenidoantimonate and tetraethylenepentamine mixed ligands. Monatsh Chem 148, 209–216 (2017). https://doi.org/10.1007/s00706-016-1777-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00706-016-1777-8