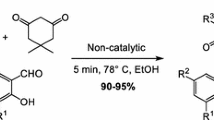
Abstract
Solvent-free sodium acetate catalyzed multicomponent reaction of isatins, malononitrile, and dimedone initiated by grinding in mortar results in the fast and efficient formation of substituted spirooxindoles in 90–99 % yields. The developed solvent-free fast multicomponent approach to the substituted spirooxindoles—the pharmacologically perspective substances with spasmolytic, diuretic, anticoagulant, anticancer, and antianaphylactic activities—is beneficial from the viewpoint of diversity-oriented large-scale processes and represents fast efficient and environmentally benign solvent-free synthetic concept for multicomponent reactions strategy.
Graphical abstract

Similar content being viewed by others
References
Dömling A, Ugi I (2000) Angew Chem Int Ed 39:3168
Weber L (2002) Drug Disc Today 7:143
Dömling A (2002) Curr Opin Chem Biol 6:306
Williams RM, Cox RJ (2003) Acc Chem Res 36:127
Cui C-B, Kakeya H, Osada H (1996) J Antibiot 49:832
Fischer C, Meyers C, Carreira EM (2000) Helv Chim Acta 83:1175
Alper PB, Meyers C, Lerchner A, Siegel DR, Carreira EM (1999) Angew Chem Int Ed 38:3186 (and references cited therein)
Ashimori A, Bachand B, Overmann LE, Poon DJ (1998) J Am Chem Soc 120:6477
Patchett AA, Nargund RP (2000) Ann Rep Med Chem 35:289
DeSimone RW, Currie KS, Mitchell SA, Darrow JW, Pippin DA (2004) Comb Chem High Throughput Screen 7:473
Skommer J, Wlodkowic D, Mättö M, Eray M, Pelkonen J (2006) Leukemia Res 30:322
Aramini JM, Germann MW, Huang Z (2000) Tetrahedron Lett 41:6993
Bonsignore L, Loy G, Secci D, Calignano A (1993) Eur J Med Chem 28:517
Konkoy CS, Fick DB, Cai SX, Lan NC, Keana JF (2000) Substituted 5-oxo-5,6,7,8-tetrahydro-4H-1-benzopyrans and benzothiopyrans and their use as potentiators of AMPA. PCT Int Appl WO 2000075123, Dec 14, 2000; (2000) Chem Abstr 134:29313
Konkoy CS, Fick DB, Cai SX, Lan NC, Keana JF (2004) Substituted 5-oxo-5,6,7,8-tetrahydro-4H-1-benzopyrans and benzothiopyrans and their use as potentiators of AMPA. US Pat 6800657 B2, Oct 5, 2004; (2000) Chem Abstr 134:29313
Gao S, Tsai CH, Tseng C, Yao C-F (2008) Tetrahedron 64:9143
Wang L-M, Jiao N, Qiu J, Yu J-J, Liu J-Q, Guo F-L, Liu Y (2010) Tetrahedron 66:339
Chai SJ, Lai Y-F, Xu J-C, Zheng H, Qing Zhu Q, Zhanga P-F (2010) Avd Synth Cat 353:371
Riyaz S, Naidu A, Dubey PK (2012) Lett Org Chem 9:101
Baharfar R, Azimi R (2014) Synth Commun 44:89
Kidwai M, Jahan A, Mishra NK (2012) Appl Cat A 425–426:35
Dandia A, Jain AK, Bhati DS (2011) Synth Commun 41:2905
Dandia A, Parewa V, Jain AK, Rathore KS (2011) Green Chem 13:2135
Saha M, Das B, Pal AK (2013) C R Chemie 16:1078
Elinson MN, Ilovaisky AI, Dorofeev AS, Merkulova VM, Stepanov NO, Miloserdov FM, Ogibin YN, Nikishin GI (2007) Tetrahedron 63:10543
Elinson MN, Ilovaisky AI, Merkulova VM, Zaimovskaya TA, Nikishin GI (2012) Mendeleev Commun 22:143
Sheldon RA (2000) Pure Appl Chem 72:1233
Hosseini-Sarvari M, Mina Tavakolian M (2012) Comb Chem High Throughput Screen 15:826
Elinson MN, Ilovaisky AI, Merkulova VM, Belyakov PA, Chizhov AO, Nikishin GI (2010) Tetrahedron 66:4043
Elinson MN, Medvedev MG, Ilovaisky AI, Merkulova VM, Zaimovskaya TA, Nikishin GI (2013) Mendeleev Commun 23:94
Elinson MN, Nasybullin RF, Ryzhkov FV, Zaimovskaya TA, Egorov MP (2014) Monatsh Chem 145:605
Elinson MN, Nasybullin RF, Ryzhkov FV, Egorov MP (2014) C R Chimie 17:437
Elinson MN, Ryzhkov FV, Merkulova VM, Ilovaisky AI, Nikishin GI (2014) Heterocycl Commun 20:281
Elinson MN, Nasybullin RF, Ryzhkov FV, Zaimovskaya TA, Nikishin GI (2015) Monatsh Chem 146:631
Elinson MN, Ryzhkov FV, Vereshchagin AN, Gorbunov SV, Egorov MP (2015) C R Chimie 18:540
Elinson MN, Ryzhkoy FV, Zaimoyskaya TA, Egorov MP (2015) Mendeleev Commun 25:185
Patai S, Israeli Y (1960) J Chem Soc 2025
Acknowledgments
The authors gratefully acknowledge the financial support of the Russian Foundation for Basic Research (Project No. 13-03-00096a).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Elinson, M.N., Ryzhkov, F.V., Zaimovskaya, T.A. et al. Solvent-free multicomponent assembling of isatins, malononitrile, and dimedone: fast and efficient way to functionalized spirooxindole system. Monatsh Chem 147, 755–760 (2016). https://doi.org/10.1007/s00706-015-1617-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00706-015-1617-2