Abstract
The urea–choline chloride-based deep eutectic solvent was found to be an efficient catalyst and reaction media for the additive-free synthesis of imines (Schiff bases) and hydrobenzamides by the reaction of aldehydes with amines and ammonia in good to high yields. Outstanding features of this protocol were the general and atom-economical reaction, absence of external catalysts and additives, simple workup, and availability and recycling of urea–choline chloride as a green solvent.
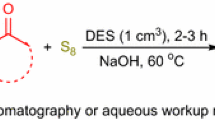
Graphical abstract

Similar content being viewed by others
References
Abbott AP, Davies DL, Rasheed RK, Tambyrajah V (2003) Chem Commun 70
Smith EL, Abbott AP, Ryder KS (2014) Chem Rev 114:11060
Tang B, Ho Row K (2013) Monatsh Chem 144:1427
Zhang Q, Vigier KDO, Royer S, Jérôme F (2012) Chem Soc Rev 41:7108
Singh B, Lobo H, Shankarling G (2011) Catal Lett 141:178
Wang P, Ma F-P, Zhang Z-H (2014) J Mol Liq 198:259
Hayyan A, Ali Hashim A, Hayyan M, Mjalli FS, Al Nashef IM (2014) J Clean Prod 65:246
Kailas Sanap A, Shankarling GS (2014) Catal Commun 49:58
Kumar D, Kommi DN, Bollineni N, Patela AR, Chakraborti AK (2012) Green Chem 14:2038
Kumar D, Sonawane M, Pujala B, Jain VK, Bhagat S, Chakraborti AK (2013) Green Chem 15:2872
Layer RW (1963) Chem Rev 63:489
Roy SR, Jadhavar PS, Seth K, Sharma KK, Chakraborti AK (2011) Synthesis 2261
Thomas G (2000) Medicinal chemistry. Wiley, New York
Ajamian A, Gleason JL (2004) Angew Chem Int Ed 43:3754
Hart DJ, Ha DC (1989) Chem Rev 89:1447
Choudhury LH, Parvin T (2011) Tetrahedron 67:8213
Cushman M, He HM, Lin CM, Hamel E (1993) J Med Chem 36:2817
Kommi DN, Kumar D, Seth K, Chakraborti AK (2013) Org Lett 15:1158
Huang J-M, Zhang J-F, Dong Y, Gong W (2011) J Org Chem 76:3511
Williams OF, Bailar JC (1959) J Am Chem Soc 81:4464
Naka H, Koseki D, Kondo Y (2008) Adv Syn Catal 350:1901
Li T, Cui X, Sun L, Li C (2014) RSC Adv 4:33599
Fujii H, Nagamatsu K, Gunji T, Murafuji T, Abe N (2010) Heterocycles 81:2625
Greger JG, Yoon-Miller SJP, Bechtold NR, Flewelling SA, MacDonald JP, Downey CR, Cohen EA, Pelkey ET (2011) J Org Chem 76:8203
Seomoon D, Jaemyung A, Lee PH (2009) Org Lett 11:2401
Chakraborti AK, Bhagat S, Rudrawar S (2004) Tetrahedron Lett 45:7641
Azizi N, Rajabi F, Saidi MR (2004) Tetrahedron Lett 45:9233
Abbiati G, Contini A, Nava D, Rossi E (2009) Tetrahedron 65:4664
Karupaiyan S, Deshmukh B (1998) Tetrahedron 54:4375
Chou CH, Chu LT, Chiu S, Lee CF, She YT (2004) Tetrahedron 60:6581
Risse J, Scopelliti R, Severin K (2011) Organomet 30:3412
Uchida H, Tanikoshi H, Nakamura S, Reddy PY, Toru T (2003) Synlett 1117
Lozinskaya T, Proskurnina Z (2003) Russ Chem Bull 52:674
Grigorev V, Mirskov R (2001) Russ J Gen Chem 71:149
Pudovik S, Khairullin V, Chernova G (1996) Russ Chem Bull 45:1242
Azizi N, Amiri AK, Ghafuri H, Bolourtchian M (2011) Mol Divers 15:157
Azizi N, Batebi E (2012) Catal Sci Technol 2:2445
Azizi N, Batebi E, Bagherpour S, Ghafuri H (2012) RSC Adv 2:2289
Azizi N, Gholibeglo E (2012) RSC Adv 2:7413
Azizi N, Manocheri Z (2012) Res Chem Intermed 38:1495
Azizi N, Khajeh-Amiri A, Ghafuri H, Bolourtchian M, Saidi MR (2009) Synlett 2245
Acknowledgments
Financial support of this work by Chemistry and Chemical Engineering Research Center of Iran is gratefully appreciated.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Azizi, N., Edrisi, M. Deep eutectic solvent catalyzed eco-friendly synthesis of imines and hydrobenzamides. Monatsh Chem 146, 1695–1698 (2015). https://doi.org/10.1007/s00706-015-1447-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00706-015-1447-2