Abstract
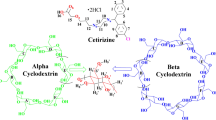
The structural aspects of the complexation of (R)- and (S)-benzhexol with β-cyclodextrin (β-CD) were explored using quantitative rotating-frame overhauser effect spectroscopy (ROESY) analysis and molecular mechanics (MM) and molecular dynamics (MD) simulations. Several modes of penetration of phenyl ring, studied by MM2 minimizations, confirmed preference for wide side entry. MD simulations were performed through wider side only and interproton distances between phenyl ring of benzhexol (BEN) and β-CD cavity obtained from lowest energy structures were used to calculate relative ROESY peak intensities. The structures for which calculated intensities were found in better agreement with experimental were then fine-tuned to obtain proposed structures. The results show that highly symmetrical cyclodextrin should be used for computational studies and kept static throughout the simulation. The energy should not be taken as the only criterion for final selection but a comparison of calculated ROESY intensity ratios with experimental ratios is better. The results also imply that ROESY peak intensity ratios can be used for quantitative purposes even with higher mixing time.
Graphical abstract










Similar content being viewed by others
References
Lehn J-M (1988) Angew Chem Int Ed 27:89
Lehn J-M (2002) Science 295:2400
Klajn R, Stoddart JF, Grzybowski BA (2010) Chem Soc Rev 39:2203
Sokolov AN, Bučar D, Baltrusaitis J, Gu SX, MacGillivray LR (2010) Angew Chem Int Ed 49:4273
Kay ER, Leigh DA, Zerbetto F (2007) Angew Chem Int Ed 46:72
Qi Z, Malo de Molina P, Jiang W, Wang Q, Nowosinski K, Schulz A, Gradzielski M, Schalley CA (2012) Chem Sci 3:2073
Schneider H-J (1991) Angew Chem Int Ed 30:1417
Webb TH, Wilcox CS (1993) Chem Soc Rev 22:383
Del Valle EMM (2004) Process Biochem 39:1033
Dodziuk H, Kozmin SKW, Ejchart A (2004) Chirality 16:90
Jullian C, Miranda S, Zapata-Torres G, Mendizábal F, Olea-Azarb C (2007) Bioorg Med Chem 15:3217
Raffaini G, Ganazzoli F, Malpezzi L, Fuganti C, Fronza G, Panzeri W, Mele A (2009) J Phys Chem B 113:9110
Jug M, Mennini N, Melani F, Maestrelli F, Mura P (2010) Chem Phys Lett 500:347
Neuhaus D, Williamson MP (2000) The nuclear overhauser effect in structural and conformational analysis, 2nd edn. Wiley, New York
Butts CP, Jones CR, Towers EC, Flynn JL, Appleby L, Barron N (2011) J Org Biomol Chem 9:177
Ali SM, Upadhyay SK (2008) Magn Reson Chem 46:676
Ali SM, Khan S, Crowyn G (2012) Magn Reson Chem 50:299
Giachetti A, Giraldo E, Ladinsky H, Montagna E (1986) Br J Pharmacol 89:83
Demarco PV, Thakkar AL (1970) J Chem Soc Chem Commun 2
Schneider H-J, Hacket F, Rüdiger V, Ikeda H (1998) Chem Rev 98:1755
Schjelderup L, Harbitz O, Groth P, Aasen AJ (1987) Acta Chem Scand B 41:356
Benesi AH, Hildebrandt JH (1949) J Am Chem Soc 71:2703
Scott RL (1956) Rec Trav Chim 75:787
Richards SA, Hollerton JC (2010) Essential practical NMR for organic chemistry. Wiley, New York
Zhao R, Tan T, Sandström C (2011) J Biol Phys 37:387
Zheng Y, Chow AHL, Haworth IS (2008) Lett Drug Des Discov 5:512
Allinger NL, Yuh YH, Lii J-H (1989) J Am Chem Soc 111:8551
Zabel V, Saenger W, Mason SA (1986) J Am Chem Soc 108:3664
Jingye L, Deyue Y, Qun C (2002) Sci China Ser B 45:73
Köhler JEH, Grczelschak-Mick N (2013) Beilstein J Org Chem 9:118
Grube A, Köck M (2007) Angew Chem Int Ed 46:2320
Chini MG, Jones CR, Zampella A, D’Auria MV, Renga B, Fiorucci S, Butts CP, Bifulco G (2012) J Org Chem 77:1489
Macura S, Farmer BTII, Brown LR (1986) J Magn Reson 70:493
Acknowledgments
Benzhexol and β-cyclodextrin were very kindly provided by Surya Pharmaceutical Ltd, Chandigarh, India, and Geertrui Haest, Cerestar Cargill, Belgium, respectively. We are grateful to the Mr. Avtaar Singh, Sophisticated Analytical Instrumentation Facility, Punjab University, Chandigarh for assistance in providing NMR spectra. Shazia Shamim is thankful to UGC, Government of India, for the financial assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ali, S.M., Shamim, S. Complexation of (RS)-benzhexol with β-cyclodextrin: structure elucidation of diastereomeric complexes by use of quantitative 1H-1H ROESY and computational methods. Monatsh Chem 146, 283–290 (2015). https://doi.org/10.1007/s00706-014-1324-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00706-014-1324-4