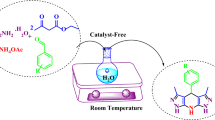
Abstract
An expedient and eco-friendly method for synthesis of quinoxalines and related compounds by the reaction of various 1,2-diketones with 1,2-diamines “on water” has been developed. The method gives very good to excellent yield of the products within 0.5–4 h.
Graphical abstract



Similar content being viewed by others
References
He W, Meyers MR, Hanney B, Spada AP, Blider G, Galzeinski H, Amin D, Needle S, Page K, Jayyosi Z, Perrone MH (2003) Bioorg Med Chem Lett 13:3097
Kim YB, Kim YH, Park JY, Kim SK (2004) Bioorg Med Chem Lett 14:541
Park Y-S, Shin W-S, Kim S-K (2008) J Antimicrob Chemother 61:163
Cholo MC, Steel HC, Fourie PB, Germishuizen WA, Anderson R (2012) J Antimicrob Chemother 67:290
Chen B-C, Zhao R, Bednarz MS, Wang B, Sundeen JE, Barrish JC (2004) J Org Chem 69:977
Chen P, Norris D, Iwanowicz EJ, Spergel SH, Lin J, Gu HH, Shen Z, Wityak J, Lin T-A, Pang S, De Fex HF, Pitt S, Shen DR, Doweyko AM, Bassolino DA, Roberge JY, Poss MA, Chen B-C, Schieven GL, Barrish JC (2002) Bioorg Med Chem Lett 12:1361
Dailey S, Feast WJ, Peace RJ, Sage IC, Tilla S, Wooda EL (2001) J Mater Chem 11:2238
Nageswar YVD, Reddy KHV, Ramesh K, Murthy SN (2013) Org Prep Proced Int 45:1
Dhakshinamoorthy A, Kanagaraj K, Pitchumani K (2011) Tetrahedron Lett 52:69
Chavan H, Adsul L, Bandgar BP (2011) J Chem Sci 123:477
Das PJ, Sarkar S (2011) Int J Chem Res 3:56
Gu X, Li X, Chai Y, Yang Q, Li P, Yao Y (2011) Green Chem 13:1640
Sajjadifar S, Zolfigol MA, Mirshokraie SA, Miri S, Louie O, Nezhad ER, Karimian S, Darvishi G, Donyadari E, Farahmand S (2012) Am J Org Chem 2:97
Beheshtiha YS, Heravi MM, Amrollah M, Saeedi M, Fallah A (2012) Chem Sci Trans 1:134
Khaksar S, Rostamnezhad F (2012) Bull Korean Chem Soc 33:2581
Soleymni R, Niakan N, Tayeb S, Hakimi S (2012) Orient J Chem 28:687
Ruiz DM, Autino JC, Quaranta N, Vazquez PG, Romanelli GP (2012) Sci World J 1
Sadeghi B, Karimi F (2013) Iran J Catal 3:1
Hakimia F, Mirjalilib BBF (2013) Curr Chem Lett 2:1
Kadam HK, Khan S, Kunkalkar RA, Tilve SG (2013) Tetrahedron Lett 54:1003
Duval RA, Lever JR (2010) Green Chem 12:304
Li C-J, Chen L (2006) Chem Soc Rev 35:68
Hasaninejad A, Zare A, Zolfigol MA, Shekouhy M (2009) Synth Commun 39:569
Beheshtiha YS, Heravi MM, Saeedi M, Karimi N, Zakeri M, Hossieni TN (2010) Synth Commun 40:1216
Liu J-Y, Liu J, Wang J-D, Jiao D-Q, Liu H-W (2010) Synth Commun 40:2047
Khaksar S, Alipour M (2013) Monatsh Chem 144:395
Xu H, Deng H, Li Z, Xiang H, Zhou X (2013) Eur J Org Chem 7054
Andrade CKZ, Barreto ADFS, Silva WA (2008) Arkivoc 226
Piltan M, Moradi L, Abasi G, Zarei SA (2013) Beilstein J Org Chem 9:510
He Y, Zhang X, Cui L, Wang J, Fan X (2012) Green Chem 14:3429
Tada N, Cui L, Okubo H, Miura T, Itoh A (2010) Chem Commun 46:1772
Kumar A, Kumar M, Gupta MK, Gupta LP (2012) RSC Adv 2:8277
Liu J, Lei M, Hu L (2012) Green Chem 14:2534
Xie Z-B, Wang N, Wu M-Y, He T, Le Z-G, Yu X-Q (2012) Beilstein J Org Chem 8:534
Galletti P, Pori M, Giacomini D (2011) Eur J Org Chem 3896
Mallik AK, Pal R, Guha C, Mallik H (2012) Green Chem Lett Rev 5:321
Das Gupta A, Samanta S, Mondal R, Mallik AK (2012) Bull Korean Chem Soc 33:4239
Li C-J, Chan T-H (1997) Organic Reactions in Aqueous Media. John Wiley & Sons, New York, p 1
Niknam K, Saberi D, Mohagheghnejad M (2009) Molecules 14:1915
Karami B, Khodabakhshi S, Nikrooz M (2011) Polycycl Arom Compd 31:97
Hou J-T, Liu Y-H, Zhang Z-H (2010) J Heterocycl Chem 47:703
Sharma RK (2011) Catal Commun 12:327
Lue H-Y (2010) Austr J Chem 63:1290
Haftbaradaran F, Draper ND, Leznoff DB, Williams VE (2003) Dalton Trans 2105
Sajjadifar S, Zolfigol MA, Chehardoli G, Miri S, Moosavi P (2013) Int J ChemTech Res 5:422
Cai J–J (2008) Tetrahedron Lett 49:7386
Shi D-Q, Dou G-L (2008) Synth Commun 38:3329
Neumann H, Brennführer A, Beller M (2012) Chem Eur J 14:3645
Akkilagunta VK, Reddy VP, Kakulapati RR (2010) Synlett 17:2571
Acknowledgments
Financial assistance from the UGC-CAS and DST-PURSE programs, Department of Chemistry is gratefully acknowledged. The authors also acknowledge the DST-FIST program to the Department of Chemistry, Jadavpur University for providing the NMR spectral data. SS and AD are thankful to CSIR, New Delhi for their Research Fellowships.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Samanta, S., Das Gupta, A. & Mallik, A.K. An expedient “on-water” synthesis of quinoxalines. Monatsh Chem 145, 1669–1673 (2014). https://doi.org/10.1007/s00706-014-1242-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00706-014-1242-5