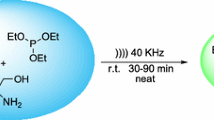
Abstract
An efficient method for the synthesis of N,N-bis(phenacyl)anilines was developed via smooth condensation of anilines with α-bromoacetophenones in the presence of sodium carbonate as acid acceptor and polyethylene glycol 400 (PEG 400) as catalyst at room temperature under solvent-free conditions by using 350 W ultrasound irradiation. In addition to experimental simplicity, the main advantages of the procedure are mild conditions, short reaction times (30–45 min), and high yields (73–83 %).
Graphical Abstract

Similar content being viewed by others
Introduction
N,N-Bis(phenacyl)anilines are of particular importance in the fine chemical industry owing to their applications as precursors of various heterocyclic compounds such as piperidine, triazepine, 1,4-dihydropyrazine, and indole [1–4]. Traditional methods for the synthesis of N,N-bis(phenacyl)aniline were achieved through the alkylation of anilines with α-bromoacetophenone, which gave low and unsatisfactory yields, and needed organic solvents and long reaction times [5–7]. In addition, the grinding method was also used in the preparation of N,N-bis(phenacyl)aniline; however, the method applied only to small-scale production, which is its main disadvantage [2].
Phase transfer catalysis (PTC) is widely applied in many industries such as fine chemicals, agrochemicals, specialty chemicals, pharmaceuticals, perfumes, flavors, dyes, and polymers, and even pollution and environmental control processes [8–10]. PEGs are soluble, recoverable, thermally stable, and inexpensive phase transfer catalysts. PEG and its many derivatives have become popular and are used in several commercial processes to replace expensive and environmentally harmful phase transfer catalysts in PTC reactions [11–13]. Recently, ultrasound-assisted solvent-free reactions have emerged as valuable tools in organic synthesis that use less organic solvent, milder conditions, shorter reaction times, and afford excellent yields with higher selectivity [14–17]. PTC and ultrasound are two clean and useful protocols in organic synthesis; ultrasonic irradiated synthesis of some interesting heterocyclic compounds under PTC has been listed in several reports [18–21]. In the framework of our investigations on the development of green chemical procedures [22, 23], we herein report a novel and environmentally safe procedure for the rapid preparation of N,N-bis(phenacyl)anilines in the presence of sodium carbonate as acid acceptor and PEG 400 as catalyst at room temperature without solvent under ultrasound irradiation (Scheme 1). This approach is designed to overcome the limitations previously encountered in the reaction.
Results and discussion
For initial optimization of the reaction conditions, a mixture of aniline (1a), α-bromoacetophenone (2a), and sodium carbonate was irradiated in the presence of PEG 400 at room temperature (Scheme 1). By increasing the irradiation power from 200 to 400 W, the reaction time of 3a decreased from 2.5 h to 50 min and the yield increased from 38 to 54 %. The data in Table 1 show that the reaction time and yield of 3a did not change when the power changed from 350 to 400 W; therefore, 350 W of ultrasonic irradiation was sufficient to promote the reaction. The best yield for 3a was obtained by ultrasonic irradiation for 50 min at room temperature and 350 W in the presence 2 mol% PEG 400. It is therefore obvious that ultrasound irradiation accelerates the condensation of aniline with α-bromoacetophenone possibly owing to the simultaneous heating and transport caused by ultrasound [24].
The catalytic activity of PEG 400 was also studied. A blank reaction was conducted with aniline, α-bromoacetophenone, and sodium carbonate in the absence of catalyst, and N,N-bis(phenacyl)aniline 3a was obtained in 11 % yield after 2.5 h. With increasing amounts of PEG 400 (1, 2, 3, 4, 5, 6 mol%), 3a was produced in 35 to 82 % and the reaction time decreased from 90 to 30 min (Table 2). The use of 5 mol% of PEG 400 was sufficient to promote the reaction, whereas a larger amount of the catalyst did not improve the results greatly. Therefore, 5 mol% of PEG 400 was chosen as the optimal catalyst amount for the synthesis of 3a.
The reactions generally gave high yields of products (Table 3). No obvious electronic effect of the substituents of the anilines was observed in the reactions because electron-donating as well as electron-withdrawing groups were well tolerated. Compound 3d was previously prepared in low and unsatisfactory yield in EtOH as solvent [5], and 3b in 64 % yield by the grinding method [2], whereas our procedure gave these N,N-bis(phenacyl)anilines in 79 and 83 % yield, respectively. Compounds 3c and 3f were previously prepared in 70 and 68 % yield, respectively, by grinding method after 1 h [2]. Our procedure gave 3c and 3f in 77 and 81 % yield, respectively, within 40 min.
In summary, we developed an efficient method for the synthesis of bis(phenacyl)anilines via condensation of anilines with α-bromoacetophenones in the presence of sodium carbonate as acid acceptor and PEG 400 as catalyst at room temperature under solvent-free conditions by using 350 W ultrasound irradiation.
Experimental
All chemicals and reagents were purchased from commercial sources and used without further purification. Melting points were determined on an X-5 instrument. IR spectra were performed as KBr pellets on a Bruker VERTEX 70 spectrophotometer. NMR spectra were measured on a Bruker AVANCE 400 spectrometer. MS spectra were recorded on a ZAB-HS and ESQUIRE 6000 mass spectrometer. Ultrasonication was performed in a GEX750-5C ultrasonic processor equipped with a 3-mm-wide and 140-mm-long titanium alloy probe that was immersed directly into the reaction mixture. In all reactions the tip of the sonotrode was located in the same position just under the liquid surface in order to obtain optimal sonication and reproducible results. The operating frequency was 24 kHz and the output power was 0–750 W through manual adjustment. The reactions were carried out in a four-neck pear-shaped flask of 50 cm3 capacity in the open air. The temperature was controlled by a Büchi B-491 water bath at room temperature (25 ± 1 °C). Elemental analyses were carried out on a Carlo Erba 1106 elemental analysis instrument.
General procedure for the preparation of the N,N-bis(phenacyl)anilines 3a–3i
A mixture of the aniline (10.0 mmol), α-bromoacetophenone (20 mmol), sodium carbonate (10.0 mmol), and 0.2 g PEG 400 (0.5 mmol) was irradiated under an ultrasonic processor at room temperature and 350 W in the open air. The reactions were completed within 30–45 min. The product was washed or recrystallized from 15 cm3 EtOH to afford the N,N-bis(phenacyl)aniline. All products were identified by their melting points, IR, 1H NMR spectra, and 13C NMR spectra and comparison with reported data [2, 5] (see also Table 3).
N,N-Bis(4-methylphenacyl)-p-chloroaniline (3h, C24H22ClNO2)
Light yellow crystals; M.p.: 178.5–181.3 °C; IR (KBr): \( \bar{v} \) = 3,033, 1,663, 1,510, 1,479, 1,375, 1,112, 792 cm−1; 1H NMR (400 MHz, CDCl3): δ = 2.35 (s, 6H, CH3), 4.92 (s, 4H, N-CH2), 6.45 (d, J = 9.2 Hz, 2H, Ar–H), 7.09–7.11 (m, 2H, Ar–H), 7.49–7.53 (m, 4H Ar–H), 7.61–7.65 (m, 2H, Ar–H), 8.00–8.02 (m, 4H Ar–H) ppm; 13C NMR (100 MHz, CDCl3): δ = 58.3, 113.5, 120.3, 128.2, 128.9, 130.4, 135.6, 141.2, 149.5, 195.8 ppm; MS: m/z = 391 (M+).
N,N-Bis(4-chlorophenacyl)-p-chloroaniline (3i, C22H16Cl3NO2)
Light yellow crystals; M.p.: 181.5–182.7 °C; IR (KBr): \( \bar{v} \) = 3,033, 1,663, 1,510, 1,479, 1,375, 1,112, 792 cm−1; 1H NMR (400 MHz, CDCl3): δ = 4.95 (s, 4H, N-CH2), 6.51 (d, J = 8.8 Hz, 2H, Ar–H), 7.08–7.10 (m, 2H, Ar–H), 7.43–7.58 (m, 4H Ar–H), 7.61–7.72 (m, 2H, Ar–H), 8.00–8.08 (m, 4H Ar–H) ppm; 13C NMR (100 MHz, CDCl3): δ = 58.3, 113.5, 120.3, 128.2, 128.9, 130.4, 135.6, 141.2, 149.5, 195.8 ppm; MS: m/z = 431 (M+).
References
Ravindran G, Muthusubramanian S, Perumal S (2008) Arkivoc 57
Ravindran G, Muthusubramanian S, Selvaraj S, Perumal S (2007) J Heterocycl Chem 44:133
Fourrey JL (1987) J Chem Soc Perkin Trans 1:1841
Jumina (2005) Indo J Chem 5:156
Almstrom GK (1916) Liebigs Ann Chem 411:350
Lellman E, Donner A (1890) Chem Ber 23:166
Mohlau R (1881) Chem Ber 14:171
Zaidman B, Sasson Y, Neumann R (1985) Ind Eng Chem Des Dev 44:390
Stinson SC (1986) Chem Eng News 27
Halpern M (1996) Catal Commun 2:1
Baj S, Siewniak A (2007) Appl Catal A 321:175
Neumann R, Sasson Y (1984) J Org Chem 49:3448
Chen J, Spear KS, Huddleston JG, Rogers RD (2005) Green Chem 7:64
Mojtahedi MM, Javadpour M, Abaee MS (2008) Ultrason Sonochem 15:828
Kaboudin B, Fallahi M (2011) Tetrahedron Lett 52:4346
Ji SJ, Shen ZL, Gu DG, Huang XY (2005) Ultrason Sonochem 12:161
Khalaj A, Doroudi A, Adibpour N, Araghi GM (2009) Asian J Chem 21:997
Xue SJ, Ke SY, Wei TB, Duan LP, Guo YL (2004) J Chin Chem Soc 51:1013
Bhatkhande BS, Samant SD (1998) Ultrason Sonochem 5:7
Li JT, Liu XF (2008) Ultrason Sonochem 15:330
Gao W, Zheng M, Li Y (2011) Beilstein J Org Chem 7:1533
He JY, Xin HX, Yan H, Song XQ, Zhong RG (2011) Ultrason Sonochem 18:466
He JY, Xin HX, Yan H, Song XQ, Zhong RG (2011) Helv Chim Acta 94:159
Cravotto G, Cintas P (2006) Chem Soc Rev 35:180
Nagy J, Madarász Z, Rapp R, Szöllösy A, Nyitrai J, Döpp D (2000) J Prakt Chem 342:281
Acknowledgments
We acknowledge Shijiazhuang University for financial support of this work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
He, J., Shi, L., Liu, S. et al. Ultrasound-mediated synthesis of N,N-bis(phenacyl)aniline under solvent-free conditions. Monatsh Chem 145, 213–216 (2014). https://doi.org/10.1007/s00706-013-0978-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00706-013-0978-7