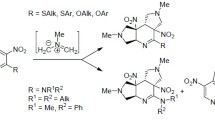
Abstract
Thermally initiated combined intra-intermolecular criss-cross cycloadditions of substituted nonsymmetrical allenyl azines with isocyanate or isothiocyanate dipolarophiles were investigated. Substituted 1,3,10-triazatricyclo[5.2.1.04,10]dec-6-en-2-ones or -dec-6-ene-2-thiones were obtained as the main products in moderate to very good yields. The thermal stabilities of the tricyclic adducts in reactions with the competing reactive dipolarophile dimethyl acetylenedicarboxylate were studied and are discussed. In some cases, substituted 5,6-dihydro-4H-pyrrolo[1,2-b]pyrazoles were found as side products. This reaction showed high atom economy and in all cases we observed the presence of one diastereoisomer and regioisomer only, which was clearly revealed by crystallographic analysis.
Graphical abstract






Similar content being viewed by others
References
Potáček M, Marek R, Žák Z, Trottier J, Janoušek Z, Viehe HG (1993) Tetrahedron Lett 34:8341
Zachová H, Man S, Nečas M, Potáček M (2005) Eur J Org Chem 2548
Man S, Kulhánek P, Potáček M, Nečas M (2002) Tetrahedron Lett 43:6431
Galeta J, Man S, Potáček M (2009) Arkivoc vi:245
Kulhánek P, Potáček M, Koča J (2004) Collect Czech Chem Commun 69:231
Man S, Bouillon JP, Nečas M, Potáček M (2004) Tetrahedron Lett 45:9419
Man S, Nečas M, Bouillon JP, Baillia H, Harakat D, Potáček M (2005) Tetrahedron 61:2387
Galeta J, Man S, Bouillon JP, Potáček M (2011) Eur J Org Chem 392
Man S, Nečas M, Bouillon JP, Portella C, Potáček M (2006) Eur J Org Chem 3473
Galeta J, Man S, Valoušková A, Potáček M (2013) Chem Pap 67:40
Henkel JG, Hane JT, Gianutsos G (1982) J Med Chem 25:51
Zah J, Terre’Blanche G, Erasmus E, Malan SF (2003) Bioorg Med Chem 11:3569
Blanpied TA, Clarke RJ, Johnson JW (2005) J Neurosci 25:3312
Furber M, Alcaraz L, Bent JE, Beyerbach A, Bowers K, Braddock M, Caffrey MV, Cladingboel D, Collington J, Donald DK, Fagura M, Ince F, Kinchin EC, Laurent C, Lawson M, Luker TJ, Mortimore MMP, Pimm AD, Riley RJ, Roberts N, Robertson M, Theaker J, Thorne PV, Weaver R, Webborn P, Willis P (2007) J Med Chem 50:5882
Miyazaki A, Tsuda Y, Fukushima S, Yokoi T, Vántus T, Bökönyi G, Szabó E, Horváth A, Kéri G, Okada Y (2008) J Med Chem 51:5121
Cady SD, Wang J, Wu Y, DeGrado WF, Hong M (2011) J Am Chem Soc 133:4274
Fort RC, Schleyer P von R (1964) Chem Rev 64:277
van Bommel KJC, Metselaar GA, Verboom W, Reinhoudt DN (2001) J Org Chem 66:5405
Jeong HY (2002) Thin Solid Films 417:171
Morcombe CR, Zilm KW (2003) J Magn Reson 162:479
Lenzke K, Landt L, Hoener M, Thomas H, Dahl JE, Liu SG, Carlson RMK, Möller T, Bostedt C (2007) J Chem Phys 127:084320
Tominaga M, Masu H, Azumaya I (2009) J Org Chem 74:8754
Lim H, Chang JY (2010) Macromolecules 43:6943
Tiwari RN, Tiwari JN, Chang L (2010) Chem Eng J 158:641
SHELXTL version 5.10 (1997) Bruker AXS. Madison, WI
Acknowledgments
The research was supported by the Grant Agency of the Czech Republic, grant no. 203/09/1345. We are grateful to Marek Nečas for crystallographic measurements and to Lukáš Maier for NMR measurements on the Bruker Avance 500.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Galeta, J., Man, S., Valoušková, A. et al. Homoallenyl azines in criss-cross cycloaddition reactions. Monatsh Chem 144, 205–216 (2013). https://doi.org/10.1007/s00706-012-0865-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00706-012-0865-7