Abstract
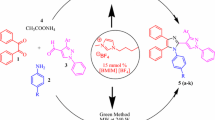
Trichloroisocyanuric acid was found to be a highly efficient homogeneous catalyst for synthesis of imidazoles via one-pot, three-component condensation of aldehydes with benzil and ammonium acetate. The valuable advantages of this method are high yields of products, easy work-up and purification procedure, and use of a commercially available, inexpensive, and non-volatile catalyst.
Graphical abstract



Similar content being viewed by others
References
Zhu J, Bienayme H (2005) Multicomponent reactions. Wiley–VCH, Weinheim
Domling A (2006) Chem Rev 106:17
Weber L (2002) Drug Discov Today 7:143
Tempest PA (2005) Curr Opin Drug Discov Dev 8:776
Cook AH, Jones DG (1941) J Chem Soc 278
Heeres J, Backx LJJ, Mostmans JH, Van Cutsem J (1979) J Med Chem 22:1003
Tanigawara Y, Aoyama N, Kita T, Shirakawa K, Komada F, Kasuga M, Okumura K (1999) Clin Pharmacol Ther 66:528
Brimblecombe RW, Duncan WAM, Durant GJ, Cemmett JC, Ganellin CR, Parsons ME (1975) J Int Med Res 3:86
Dorneanu O, Popovici I, Boiculese L, Popovici I, Bosnea DJ (2003) Preventive Med 11:41
Dinh HT, Kernbaum S, Frottier J (1978) Lancet 3111:338
Lee JC, Laydon JT, McDonnell PC, Gallagher TF, Kumar S, Green D, McNulty D, Blumenthal MJ, Keys JR, Vatter SWL, Strickler JE, Mclaughlin MM, Siemens IR, Fisher SM, Livi GP, White JR, Adams JL, Young PR (1994) Nature 372:739
Takle AK, Brown MJ, Davies S, Dean DK, Francis G, Gaiba A, Hird AW, King FD, Lovell PJ, Naylor A, Reith AD, Steadman JG, Wilson DM (2006) Bioorg Med Chem Lett 16:378
de Laszlo SE, Hacker C, Li B, Kim D, MacCoss M, Mantalo N, Pivnichny JV, Colwell L, Koch GE, Cascieri MA, Hagmenn WK (1999) Bioorg Med Chem Lett 9:641
Schmierer R, Mildenberger H, Buerstell H (1987) German Patent 361464; (1988) Chem Abstr 108:37838
Maier T, Schmierer R, Bauer K, Bieringer H, Buerstell H, Sachse B (1989) US Patent 4820335; (1989) Chem Abstr 111:19494W
Antolini M, Bozzoli A, Ghiron C, Kennedy G, Rossi T, Ursini A (1999) Bioorg Med Chem Lett 9:1023
Wang L, Woods KW, Li Q, Barr KJ, McCroskey RW, Hannick SM, Gherke L, Credo RB, Hui Y-H, Marsh K, Warren R, Lee JY, Zielinsky-Mozng N, Frost D, Rosenberg SH, Sham HL (2002) J Med Chem 45:1697
Lombandine JG, Wiseman EH (1974) J Med Chem 17:1182
Wasserschied P, Keim W (2000) Angew Chem Int Ed 39:3772
Chowdhurg S, Mohan RS, Scott JL (2007) Tetrahedron 63:2363
Shaabani A, Rahmati A (2006) J Mol Catal A: Chem 249:246
Sharma SD, Hazarika P, Konwar D (2008) Tetrahedron Lett 49:2216
Sangshetti JN, Kokare ND, Kotharkara SA, Shinde DB (2008) J Chem Sci 120:463
Heravi MM, Bakhtiari K, Oskooie HA, Taheri S (2007) J Mol Catal A Chemical 263:279
Wang LM, Wang YH, Tian H, Yao YF, Shao JH, Liu B (2006) J Fluorine Chem 127:1570
Siddiqui SA, Narkhede UC, Palimkar SS, Daniel T, Loholi RJ, Srinivasan KV (2005) Tetrahedron 61:3539
Khosropour AR (2008) Ultrason Sonochem 15:659
Balalaie S, Arabanian A (2000) Green Chem 2:274
Chattaway FD, Wadmore JM (1902) J Chem Soc 81:191
Kolvari E, Ghorbani-Choghamarani A, Salehi P, Shirini F, Zolfigol MA (2007) J Iran Chem Soc 4:126
Bigdeli MA, Nemati F, Mahdavinia GH, Doostmohammadi H (2009) Chin Chem Lett 20:1275
Bigdeli MA, Jafari S, Mahdavinia GH, Hazarkhani H (2007) Catal Commun 8:1641
Veisi H, Gholbedaghi R, Malakootikhah J, Sedrpoushan A, Maleki B, Kordestani D (2010) J Heterocycl Chem 47:1398
Khazaei A, Zolfigol MA, Rostami A, Ghorbani-Choghamarani A (2007) Catal Commun 8:543
Zolfigol MA, Khazaei A, Ghorbani-Choghamarani A, Rostami A, Hajjami M (2006) Catal Commun 7:399
Shen M-G, Cai C, Yi W-B (2008) J Flurine Chem 129:541
Joshi RS, Mandhane PG, Shaikh MU, Kale RP, Gill CH (2010) Chin Chem Lett 21:429
White DM, Sonnenberg J (1964) J Org Chem 29:192
Safari J, Khalili SD, Banitaba SH (2011) Synth Commun 41:2359
Goswami S, Chakrabarty R (2011) Eur J Chem 2:410
Acknowledgments
The authors are grateful to the Research Council of Hakim Sabzevari University for the financial support of this work.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Hojati, S.F., Nezhadhoseiny, S.A. & Beykzadeh, Z. Trichloroisocyanuric acid-catalyzed one-pot synthesis of 2,4,5-trisubstituted imidazoles. Monatsh Chem 144, 387–390 (2013). https://doi.org/10.1007/s00706-012-0830-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00706-012-0830-5