Abstract
Little is known about lytic bacteriophages infecting plant-pathogenic Dickeya spp. These bacteria cause economically significant losses in arable crops and ornamental plant production worldwide. At present, there is no effective control of diseases caused by Dickeya spp. A novel bacteriophage, ϕD5, belonging to the family Myoviridae, order Caudovirales, that could be used to control these bacteria was isolated previously. This report provides information on its characterization. The ϕD5 genome consists of 155,346-bp-length double-stranded DNA with a GC content of 49.7 % and is predicted to have 196 open reading frames (ORFs) with an average length of 711 nucleotides each. The ORFs were classified into functional groups, including phage structure, packaging, DNA metabolism, regulation, and additional functions. The phage lifestyle predicted from PHACTS indicated that ϕD5 may be a lytic phage and therefore can efficiently kill plant-pathogenic Dickeya spp.
Similar content being viewed by others
Dickeya spp. (formerly Erwinia chrysanthemi or Pectobacterium chrysanthemum) together with Pectobacterium carotovorum and P. atrosepticum (formerly Erwinia carotovora subsp. carotovora and Erwinia carotovora subsp. atroseptica, respectively) are plant-pathogenic bacteria that are responsible for economically important losses in potato and other arable and ornamental crops worldwide [8, 10]. They are recognized to be among the top ten most destructive bacterial pathogens in agriculture [6]. In the past, Dickeya spp. were associated mainly with crop diseases in tropical and subtropical regions and were rarely isolated from diseased plants cultivated in Western and Northern Europe [5]. However, recently, there has been an increase in potato crop infection in Europe attributed to Dickeya spp., and especially to the presence and spread of a new virulent species named D. solani [11]. D. solani has been reported in several countries in Western Europe, including France, the Netherlands, Belgium, Poland, Germany, Spain, Sweden, Finland, and the United Kingdom, as well as in Israel and Georgia [11].
At present, there is no effective control of Dickeya spp. diseases, whether based on physical, chemical or biological measures under field conditions [3]. There are also no immune crop cultivars readily available on the market. Current control relies solely on measures in crop production that avoid or reduce the risks of contamination of the propagative material – measures that are far from being reliable [3].
Bacteriophages are a promising and environmentally friendly substitute to supplement present control strategies to protect growing plants as well as harvested crops against plant-pathogenic bacteria [7]. Their potential as biological control agents has been evaluated in different studies involving various plant pathogens [6]. Recently, Adriaenssens and co-workers presented a genetic and proteomic study on the first D. solani phage, named LIMEstone [1] and we presented data on isolation and characterization of several lytic bacteriophages against different Dickeya species [4].
Despite the growing interest in using bacteriophages to control Dickeya species, in general, there is still very little information on their genome organization and structure. Data on phage genomics may facilitate development of biological control procedures. For example, the understanding of the genetic background of the phage lifestyle can help in designing better biological control applications, and new delivery strategies may support longer stability of phages in the environment.
So far, there is only one Dickeya spp. phage genome available in the GenBank database (NC019925), and this phage has a very restricted host range, able to infect D. solani only [1]. In contrast, bacteriophage ϕD5, despite its morphological similarity (both phages belong to the family Myoviridae), has a broad host range, larger genome, and higher GC content in comparison with LIMEstone phage [4]. Due to its broad host range, phage ϕD5 appears to be a very good candidate as biological control agent.
The ϕD5 phage was isolated in our previous study from arable soil in the Pomorskie region in Poland, primarily using D. solani (strain IPO2222) as a host [4]. It has been characterized in full for its morphologic and phenotypic features. Host-range experiments have showed that it can infect/lyse not only D. solani but also other Dickeya spp., including D. dianthicola, D. zeae, D. dadantii and D. chrysanthemi [4]. Transmission electron microscopy as described by Czajkowski et al. [4] revealed that phage ϕD5 belongs to the family Myoviridae and the order Caudovirales, with a contractive tail (ca. 140 nm in length) and an icosahedral head (ca. 100 nm in diameter).
To obtain a high phage titer (ca. 1013–1014 plaque-forming units [pfu] ml−1) for genomic DNA purification, phage ϕD5 was enriched in D. solani IPO2222 [9] cultures as described previously [2]. After enrichment, bacterial cells were removed by centrifugation (8000×g, 20 min), and the resulting phage suspension (ca. 50 ml) was filtered using a 0.22-µm membrane filter to remove bacterial debris. The phage suspension was treated with DNase I (Sigma-Aldrich; final concentration, 0.5 mg ml−1) for 60 min at 37 °C with shaking (100 rpm) to digest the bacterial DNA. Phage particles were further purified and concentrated via ultracentrifugation (35,000×g, 4 °C) in a CsCl (Sigma) gradient with densities of 1.4 to 1.7 g ml−1 for 3 h following by a second round of equilibrium gradient ultracentrifugation (35,000×g, 4 °C) in 1.4 g ml−1 CsCl for 18 h. The resulting high-titer phage suspension (ca. 1.5–2 ml) was dialyzed to remove CsCl at 4 °C in CsCl solutions in water in descending CsCl concentrations (60 % to 10 % CsCl) for 2 h. The ϕD5 phage genomic DNA was isolated as described previously [2].
Phage ϕD5 genomic DNA was sequenced using the Illumina technology and re-assembled de novo at Baseclear, The Netherlands (www.baseclear.com). Structural and functional annotation was obtained from the IGS Annotation Service (Institute for Genome Sciences, University of Maryland School of Medicine automated pipeline http://ae.igs.umaryland.edu/cgi/index.cgi) and from RAST (Rapid Annotation using Subsystem Technology, accessed via the internet http://rast.nmpdr.org/). The ϕD5 genome was mapped and annotated using available phage genome sequences deposited in GenBank (http://www.ncbi.nlm.nih.gov/genbank/). The lifestyle of phage ϕD5 (lytic or temperate) was predicted using the PHACTS program (http://www.phantome.org/PHACTS/online.htm).
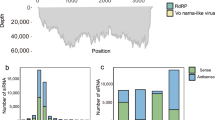
The genome sequence of phage ϕD5 contains 155,346 bp, 196 predicted open reading frames (ORFs; predicted protein-encoding genes [PEGs]) and an average GC content of 49.7 %. The average gene length was predicted to be 711 nucleotides, and 89.9 % of the genome consists of coding regions. Of the 196 putative PEGs, 50 PEGs (25.5 %) have an assigned function, 42 (21.4 %) were unclassified with no assigned category, and eight (4.1 %) PEGs have unknown function. The rest were classified as (conserved) hypothetical proteins. Functional grouping of predicted ORFs (and PEGs) revealed that two ORFs are associated with nucleotide metabolism, one with energy metabolism, seven with transport and binding proteins, nine with DNA metabolism, three with transcription, one with regulatory functions, four with the cell envelope, six with cellular processes, and four with mobile and extrachromosomal elements (Supplementary Tab. 1). The ϕD5 genome encodes only one tRNA, and for the majority of genes (94.4 %), the start codon for transcription is ATG, whereas GTG and TTG are start codons in only 4.1 % and 1.5 % phage genes, respectively. The functions of the majority of phage ϕD5 genes could not be identified, probably due to the lack of annotation data on phages present in the majority of international genome sequence databases (Fig. 1).
The genome of the bacteriophage ϕD5 (155,346 bp). Structural and functional annotation was obtained from the IGS Annotation Service (Institute for Genome Sciences, University of Maryland School of Medicine automated pipeline http://ae.igs.umaryland.edu/cgi/index.cgi) and from RAST (Rapid Annotation using Subsystem Technology, accessed via the internet http://rast.nmpdr.org/). ORFs coding for proteins involved in DNA/RNA transcription, translation and metabolism are marked in green, ORFs coding for proteins involved in bacteriophage particle assembly are marked in yellow, and ORFs coding for enzymes involved in metabolism are marked in red. The tRNA is marked with a blue square. Arrows indicate the direction of transcription and translation. The ORFs coding for hypothetical and conserved hypothetical proteins are not shown on the map. The figure was generated using the genome visualization program SnapGene ver. 2.3.4 (http://www.snapgene.com/)
To our knowledge, this is the first report of a broad-host-range Dickeya spp.-infecting bacteriophage and its complete genome sequence, and it is the first genome sequence of a Dickeya spp. bacteriophage isolated in Poland. This sequence will provide useful fundamental information for advanced molecular research on the infection/interaction of phage ϕD5 and its Dickeya spp. host.
References
Adriaenssens EM, Van Vaerenbergh J, Vandenheuvel D, Dunon V, Ceyssens PJ, De Proft M, Kropinski AM, Noben JP, Maes M, Lavigne R (2012) T4-related bacteriophage LIMEstone isolates for the control of soft rot on potato caused by ‘Dickeya solani’. PLoS One 7:e33227
Clokie MRJ, Kropinski AM (2009) Bacteriophages: methods and protocols, vol 1., Isolation, characterization, and interactions. Series: Methods in molecular biology, vol 501. Humana Press, Springer Science + Business Media, LLC, New York, NY
Czajkowski R, Pérombelon MCM, van Veen JA, van der Wolf JM (2012) Control of blackleg and tuber soft rot of potato caused by Pectobacterium and Dickeya species: a review. Plant Pathol 60:999–1013
Czajkowski R, Ozymko Z, Lojkowska E (2013) Isolation and characterization of novel soilborne lytic bacteriophages infecting Dickeya spp. biovar 3 (‘D. solani’). Plant Pathol. doi:10.1111/ppa.12157
Janse JD, Ruissen MA (1988) Characterization and classification of Erwinia chrysanthemi strains from several hosts in The Netherlands. Phytopathology 78:800–808
Mansfield J, Genin S, Magori S, Citovsky V, Sriariyanum M, Ronald M, Dow MAX, Verdier V, Beer SV, Machado MA, Toth IAN, Salmond G, Foster GD (2012) Top 10 plant pathogenic bacteria in molecular plant pathology. Mol Plant Pathol 13:614–629
Okabe N, Goto M (1963) Bacteriophages of plant pathogens. Annu Rev Phytopathol 1:397–418
Pérombelon MCM (2002) Potato diseases caused by soft rot Erwinias: an overview of pathogenesis. Plant Pathol 51:1–12
Sławiak M, van Beckhoven JRCM, Speksnijder AGCL, Czajkowski R, Grabe G, van der Wolf JM (2009) Biochemical and genetical analysis reveal a new clade of biovar 3 Dickeya spp. strains isolated from potato in Europe. Eur J Plant Pathol 125:245–261
Toth IK, van der Wolf JM, Saddler G, Lojkowska E, Hélias V, Pirhonen M, Tsror L, Elphinstone JG (2011) Dickeya species: an emerging problem for potato production in Europe. Plant Pathol 60:385–399
Van der Wolf JM, Nijhuis EH, Kowalewska MJ, Saddler GS, Parkinson N, Elphinstone JG, Pritchard L, Toth IK, Lojkowska E, Potrykus M, Waleron M, de Vos P, Cleenwerck I, Pirhonen M, Garlant L, Hélias V, Pothier JF, Pflüger V, Duffy B, Tsror L, Manulis S (2013) Dickeya solani sp. nov., a pectinolytic plant pathogenic bacterium isolated from potato (Solanum tuberosum). Int J Syst Evol Microbiol 64:768–774
Acknowledgements
This work was financially supported by the National Science Centre, Poland (Narodowe Centrum Nauki, Polska) via a postdoctoral research grant FUGA1 (DEC-2012/04/S/NZ9/00018) to R. C. We are grateful to the whole IGS Annotation Engine team at the Institute for Genome Sciences, University of Maryland School of Medicine, Baltimore, MD, USA, for providing us with the excellent, free-of-charge genome annotation service (within IGS Annotation Engine http://ae.igs.umaryland.edu/cgi/index.cgi). The authors would like to express their gratitude to M. Perombelon (ex. SCRI, Scotland) and D. Krzyzanowska (University of Gdansk, Poland) for their valuable comments on the manuscript and their editorial work.
Author information
Authors and Affiliations
Corresponding author
Additional information
The nucleotide sequence accession number
The complete genome sequence of bacteriophage ϕD5 is available in the GenBank (https://www.ncbi.nlm.nih.gov/genbank/) database under accession number KJ716335.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Czajkowski, R., Ozymko, Z., Zwirowski, S. et al. Complete genome sequence of a broad-host-range lytic Dickeya spp. bacteriophage ϕD5. Arch Virol 159, 3153–3155 (2014). https://doi.org/10.1007/s00705-014-2170-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00705-014-2170-8