Abstract
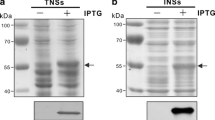
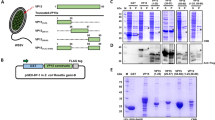
The icp11 gene encoding the highly abundant DNA mimic protein of white spot syndrome virus (WSSV) was cloned into the pTYB1 and pGEX-6P-1 expression vectors and introduced into E. coli by transformation. After induction, C-terminally intein-tagged ICP11 (ICP11-intein) and N-terminally glutathione-S-transferase (GST)-tagged ICP11 (GST-ICP11) proteins with molecular masses of 64 and 35 kDa were obtained. These proteins were purified by SDS-PAGE and used for immunization of Swiss mice for monoclonal antibody (MAb) production. Two MAbs specific for ICP11 were selected; these MAbs can be used to detect natural WSSV infection in Penaeus vannamei by dot blotting, western blotting or immunohistochemistry without cross-reaction with other shrimp tissues or other common shrimp viruses. The detection sensitivity of the MAbs was approximately 0.7 fmole/spot of GST-ICP11 as determined by dot blotting. These MAbs showed stronger immunoreactivity than other MAbs from previous studies that are specific for VP28 and VP19. A combination of MAbs specific for ICP11, VP28 and VP19 increased the detection sensitivity of WSSV during early infection to a sensitivity 250 times lower than that of one-step PCR. Therefore, the MAbs specific for ICP11 could be used to confirm and enhance the detection sensitivity for WSSV infection in shrimp using various types of antibody-based assays.







Similar content being viewed by others
References
Flegel TW (2006) Detection of major penaeid shrimp viruses in Asia, a historical perspective with emphasis on Thailand. Aquaculture 258:1–33
Chang PS, Lo CC, Wang YC, Kou GH (1996) Identification of white spot syndrome associated baculovirus (WSBV) target organs in the shrimp Penaeus monodon by in situ hybridization. Dis Aquat Org 27:131–139
Takahashi Y, Itami T, Maeda M, Suzuki N, Kasornchandra J, Supamattaya K, Khongpradit R, Boonyaratpalin S, Kondo M, Kawai K, Kusuda R, Hirono I, Aoki T (1996) Polymerase chain reaction (PCR) amplification of bacilliformvirus (RV-PJ) DNA in Penaeus japonicus Bate and systemic ectodermal and mesodermal baculovirus (SEMBV) DNA in Penaeus monodon Fabricius. J Fish Dis 19:399–403
Lo CF, Ho CH, Peng SE, Chen CH, Hsu HC, Chiu YL, Chang CF, Lui KF, Su MS, Wang CH, Kou GH (1996) White spot syndrome baculovirus (WSBV) detected in cultured and captured shrimp, crabs and other arthropods. Dis Aquat Org 27:215–225
Kimura T, Yamano K, Nakano H, Momoyama K, Hiraoka M, Inouye K (1996) Detection of penaeid rod-shaped DNA virus (PRDV) by PCR. Fish Pathol 31:93–98
Durand SV, Redman RM, Mohney LL, Tang-Nelson K, Bonami JR, Lightner DV (2003) Qualitative and quantitative studies on the relative virus load of tails and heads of shrimp acutely infected with WSSV. Aquaculture 216:9–18
Kono T, Savan R, Sakai M, Itami T (2004) Detection of white spot syndrome virus in shrimp by loop-mediated isothermal amplification. J Virol Methods 115:59–65
Mekata T, Sudhakaran R, Kono T, Supamattaya K, Linh NT, Sakai M, Itami T (2009) Real-time quantitative loop-mediated isothermal amplification as a simple method for detecting white spot syndrome virus. Lett Appl Microbiol 48:25–32
Jaroenram W, Kiatpathomchai W, Flegel TW (2009) Rapid and sensitive detection of white spot syndrome virus by loop-mediated isothermal amplification combined with a lateral flow dipstick. Mol Cell Probes 23:65–70
Lo CF, Chang YS, Cheng CT, Kou GH (1998) PCR monitoring of cultured shrimp for white spot syndrome virus (WSSV) infection in growout ponds. In: Flegel TW (ed) Advances in shrimp biotechnology. National Center for Genetic Engineering and Biotechnology, Bangkok, pp 281–286
Tsai MF, Kou GH, Liu HC, Liu KF, Chang CF, Peng SE, Hsu HC, Wang CH, Lo CF (1999) Long-term presence of white spot syndrome virus (WSSV) in cultivated shrimp population without disease outbreaks. Dis Aquat Org 38:107–114
Patil R, Palaksha KJ, Anil TM, Guruchannabasavanna Patil P, Shankar KM, Mohan CV, Sripada A (2008) Evaluation of an immunodot test to manage white spot syndrome virus (WSSV) during cultivation of the giant tiger shrimp Penaeus monodon. Dis Aquat Org 79:157–161
Nadala ECB, Loh PC (2000) Dot blot enzyme immunoassays for the detection of white spot virus and yellow-head virus of penaeid shrimp. J Virol Methods 84:175–179
You Z, Nadala ECB Jr, Yang J, van Hulten MCW, Loh PC (2002) Production of polyclonal antiserum specific to the 27.5 kDa envelope protein of white spot syndrome virus. Dis Aquat Org 51:77–80
Poulos BT, Pantoja CR, Bradley-Dunlop D, Aguilar J, Lightner DV (2001) Development and application of monoclonal antibodies for the detection of white spot syndrome virus of penaeid shrimp. Dis Aquat Org 47:13–23
Anil TM, Shankar KM, Mohan CV (2002) Monoclonal antibodies developed for sensitive detection and comparison of white spot syndrome virus isolates in India. Dis Aquat Org 51:67–75
Liu W, Wang YT, Tian DS, Yin ZC, Kwang J (2002) Detection of white spot syndrome virus (WSSV) of shrimp by means of monoclonal antibodied (MAbs) specific to an envelope protein (28 kDa). Dis Aquat Org 49:11–18
Chaivisuthangkura P, Tangkhabuanbutra J, Longyant S, Sithigorngul W, Rukpratanporn S, Menasveta P, Sithigorngul P (2004) Monoclonal antibodies against a truncated viral envelope protein (VP28) can detect white spot syndrome virus (WSSV) infections in shrimp. Sci Asia 30:359–363
Chaivisuthangkura P, Longyant S, Rukpratanporn S, Srisuk C, Sridulyakul P, Sithigorngul P (2010) Enhanced white spot syndrome virus (WSSV) detection sensitivity using monoclonal antibody specific to heterologously expressed VP19 envelope protein. Aquaculture 299:15–20
Shih HH (2004) Neutralization of white spot syndrome virus by monoclonal antibodies against viral envelope proteins. Taiwania 49:159–165
Powell JWB, Berge EJ, Browdy CL, Shepard EF (2006) Efficiency and sensitivity determination of Shrimple®, an immunochromatographic assay for white spot syndrome virus (WSSV), using quantitative realtime PCR. Aquaculture 257:167–172
Sithigorngul W, Rukpratanporn S, Pecharaburanin N, Longyant S, Chaivisuthangkura P, Sithigorngul P (2006) A simple and rapid immunochromatographic test strip for detection of white spot syndrome virus (WSSV) of shrimp. Dis Aquat Org 72:101–106
Sithigorngul P, Rukpratanporn S, Chaivisuthangkura P, Sridulyakul P, Longyant S (2011) Simultaneous and rapid detection of white spot syndrome virus and yellow head virus infection in shrimp with a dual immunochromatographic strip test. J Virol Methods 173:85–91
Huang C, Zhang X, Lin Q, Xu X, Hew CL (2002) Characterization of a novel envelope protein (VP281) of shrimp white spot syndrome virus by mass spectrometry. J Gen Virol 83:2385–2392
Zhang X, Huang C, Tang X, Zhuang Y, Hew CL (2004) Identification of structural proteins from shrimp white spot syndrome virus (WSSV) by 2DE-MS. Proteins 55:229–235
Tsai JM, Wang HC, Leu JH, Hsiao HH, Wang AH, Kou GH, Lo CF (2004) Genomic and proteomic analysis of thirty-nine structural proteins of shrimp white spot syndrome virus. J Virol 78:11360–11370
Xie X, Xu LM, Yang F (2006) Proteomic analysis of the major envelope and nucleocapsid proteins of white spot syndrome virus. J Virol 80:10615–10623
Leu JH, Yang F, Zhang X, Xu X, Kou GH, Lo CF (2009) Whispovirus. Curr Top Microbiol Immunol 328:197–227
Wang HC, Wang HC, Kou GH, Lo CF, Huang WP (2007) Identification of icp11, the most highly expressed gene of shrimp white spot syndrome virus (WSSV). Dis Aquat Org 74:179–189
Wang HC, Wang HC, Leu JH, Kou GH, Wang AH, Lo CF (2007) Protein expression profiling of the shrimp cellular response to white spot syndrome virus infection. Dev Comp Immunol 31:672–686
Wang HC, Wang HC, Ko TP, Lee YM, Leu JH, Ho CH, Huang WP, Lo CF, Wang AHJ (2008) White spot syndrome virus protein ICP11: A histone-binding DNA mimic that disrupts nucleosome assembly. PNAS 105:20758–20763
Bradford MM (1976) A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Köhler G, Milstein C (1976) Derivation of specific antibody producing tissue culture and tumor lines by cell fusion. Eur J Immunol 6:511–519
Mosmann TR, Bauman R, Williamson AR (1979) Mutations affecting immunoglobulin light chain secretion by myeloma cells. I. Functional analysis by cell fusion. Eur J Immunol 9:511–516
Sithigorngul P, Chauychuwong P, Sithigorngul W, Longyant S, Chaivisuthangkura P, Menasveta P (2000) Development of a monoclonal antibody specific to yellowhead virus (YHV) from Penaeus monodon. Dis Aquat Org 42:27–34
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685
Sithigorngul P, Hajimasalaeh W, Longyant S, Sridulyakul P, Rukpratanporn S, Chaivisuthangkura P (2009) Simple immunoblot and immunohistochemical detection of Penaeus stylirostris densovirus using monoclonal antibodies to viral capsid protein expressed heterologously. J Virol methods 162:126–132
Boonsanongchokying C, Sang-oum W, Sithigorngul P, Sriurairatana S, Flegel TW (2006) Production of monoclonal antibodies to polyhedrin of monodon baculovirus (MBV) from shrimp. Sci Asia 32:371–376
Longyant S, Poyoi P, Chaivisuthangkura P, Tejankura T, Sithigorngul W, Sithigorngul P, Rukpratanporn S (2008) Specific monoclonal antibodies raised against Taura syndrome virus (TSV) capsid protein VP3 detect TSV in single and dual infections with white spot syndrome virus (WSSV). Dis Aquat Org 79:75–81
Sithigorngul P, Rukpratanporn S, Longyant S, Chaivisuthangkura P, Sithigorngul W, Menasveta P (2002) Monoclonal antibodies specific to yellow-head virus (YHV) of Penaeus monodon. Dis Aquat Org 49:71–76
Kunanopparat A, Chaivisuthangkura P, Senapin S, Longyant S, Rukpratanporn S, Flegel TW, Sithigorngul P (2011) Detection of infectious myonecrosis virus using monoclonal antibody specific to N and C fragments of the capsid protein expressed heterologously. J Virol Methods 171:141–148
Sithigorngul P, Stretton AOW, Cowden C (1991) A versatile dot-ELISA method with femtomole sensitivity for detecting small peptides. J Immunol Methods 141:23–32
Rout N, Citarasu T, Ravindran R, Murugan V (2005) Trancriptional and transitional expression profile of white spot syndrome viral (WSSV) gene in different organs of infected shrimp. Aquaculture 245:31–38
Peng SE, Lo CF, Liu KF, Kou GH (1998) The transition from pre-patent to patent infection of white spot syndrome virus (WSSV) in Penaeus monodon triggered by pereiopod excision. Fish Pathol 33:395–400
Acknowledgments
This work was financially supported by the Strategic Wisdom and Research Institute of Srinakharinwirot University and the Higher Commission of Education, Ministry of Education, by a graduate scholarship to Mrs. Ruthairat Siriwattanarat.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Siriwattanarat, R., Longyant, S., Chaivisuthangkura, P. et al. Improvement of immunodetection of white spot syndrome virus using a monoclonal antibody specific for heterologously expressed icp11. Arch Virol 158, 967–979 (2013). https://doi.org/10.1007/s00705-012-1569-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00705-012-1569-3