Abstract
rTMS is increasingly used for a variety of neuropsychiatric conditions. There are data to support ‘fast’ rTMS (≥10 Hz) having some positive effects on cognitive functioning, but a dearth of research looking at any such effects of ‘slow’ rTMS. This question is important as cognitive dysfunction accompanies many neuropsychiatric conditions and neuromodulation that potentially enhances or hinders such functioning has important clinical consequences. To determine cognitive effects of slow (≤1 Hz) rTMS, a systematic review of randomized control trials assayed cognition in neurological, psychiatric, and healthy volunteer ≤1 Hz rTMS paradigms. Both active (fast rTMS) and placebo comparators were included. 497 Records were initially obtained; 20 met inclusion criteria for evaluation. Four major categories emerged: mood disorders; psychotic disorders; cerebrovascular accidents; and ‘other’ (PTSD, OCD, epilepsy, anxiety, and tinnitus). Cognitive effects were measured across several domains: attention, executive functioning, learning, and psychomotor speed. Variability of study paradigms and reporting precluded meta-analytical analysis. No statistically significant improvement or deterioration was consistently found in any cognitive domain or illness category. These data support the overall safety of rTMS in not adversely affecting cognitive functioning. There are some data indicating that rTMS might have cognitive enhancing potential, but these are too limited at this time to make any firm conclusions, and the literature is marked by considerable heterogeneity in study parameters that hinder interpretation. Greater consensus is required in future studies in cognitive markers, and particularly in reporting of protocols. Future work should evaluate the effects of rTMS on cognitive training.
Similar content being viewed by others
Introduction
Transcranial magnetic stimulation (TMS) is a non-invasive cortical modulating tool, where a fluctuating magnetic field induces an electrical current that depolarises underlying neurons (Wassermann et al. 2008). Repetitive TMS (rTMS) can be applied as either low (≤1 Hz) or high (≥5 Hz) frequency; the former considered typically inhibitory to underlying neurons, the latter excitatory (Pell et al. 2011). The effects on distal but functionally connected regions may be more complex (Tracy et al. 2011, Tracy et al. 2014).
rTMS alters synaptic plasticity through long-term potentiation (LTP) and long-term depression (LTD) changes (Hoogendam et al. 2010); however, the underlying mechanisms of these effects are not fully understood (Pell et al. 2011; Ridding and Rothwell 2007). Rodent studies demonstrate that rTMS increases the expression of genes important for synaptic plasticity, such as c-Fos (Aydin-Abidin et al. 2008; Doi et al. 2001), but at present, data on rTMS-induced intracellular changes in gene expression, protein synthesis, or other alterations to secondary messenger signalling are largely understudied (Hulme et al. 2013).
The ability to modulate cortical activity—relatively easily, painlessly, and without the use of a general anaesthetic—has garnered significant interest concerning potential clinical application. In psychiatric populations, the utility of rTMS in depression and psychosis has been most studied, and a recent systematic review of meta-analyses supports a modest effectiveness in both of these conditions (Hovington et al. 2013). Nascent positive results have also been obtained in the treatment of anorexia nervosa (Van den Eynde et al. 2013), bulimia nervosa (Van den Eynde et al. 2010), obsessive–compulsive disorder (OCD) (Berlim et al. 2013; Greenberg et al. 1997; Mantovani et al. 2010), tinnitus (Khedr et al. 2010; Kleinjung et al. 2005; Landgrebe et al. 2013; Langguth et al. 2003), and stroke (Khedr et al. 2009; Kim et al. 2006; Takeuchi et al. 2008). However, the literature is overall marked by often conflicting results between trials and considerable methodological concerns about study size and the lack of consensus on optimal rTMS technique parameters (Tracy and David 2015).
In cognitive neuroscience, TMS has been utilised as a tool to disrupt normal cortical activity as a means of better elucidating various cognitive processes (Miniussi and Rossini 2011; Tracy et al. 2015; Wassermann et al. 2008). Typically, non-repetitive TMS is applied during the execution of a cognitive task (so-called “online TMS”), and a transient disruption of normal functioning (a “virtual lesion”) is induced allowing inferences to be made about the role of the stimulated brain area in the cognitive task (Miniussi et al. 2010; Wassermann et al. 2008). For example, Gough et al. 2005 determined that three pulses of TMS to the anterior left inferior frontal cortex (LIFC) delivered at 100 ms intervals caused a significant slowing of response in a semantic judgement task, but not in a phonological judgment task; and conversely that TMS given to the posterior LIFC caused a significant slowing of response in the phonological task, but not in the semantic one (Gough et al. 2005). The effects of offline stimulation on cognitive functioning, with task execution and TMS stimulation temporally dissociated, have also been investigated (Demirtas-Tatlidede et al. 2013; Miniussi and Rossini 2011). Studies have largely focussed on cognitive recovery after stroke, prolonged psychiatric disease, or traumatic brain injury. No conclusive evidence is currently available regarding the use of offline non-invasive brain stimulation for the rehabilitation of such neuropsychiatric disease, though undoubtedly such work is still at a nascent stage (Demirtas-Tatlidede et al. 2013).
Most data on cognitive effects of TMS in studies on participants with mental illness come from clinical trials where they are often reported as part of safety and side-effects assessments (Demirtas-Tatlidede et al. 2013). Contrary to electroconvulsive therapy (ECT) (Schulze-Rauschenbach et al. 2005), the majority of studies show that rTMS has no clear deleterious effects, though the secondary nature of such data collection means that overall there is a dearth of information on this topic (Anderson et al. 2006; Guse et al. 2010). Some clinical trials have found rTMS to be associated with improvements across several cognitive domains (Fitzgerald et al. 2009; Hoppner et al. 2003). For example, Mogg et al. (2007) found that 10 Hz rTMS led to a significant improvement in verbal learning among patients with schizophrenia, whilst Martis et al. (2003) found that 10 Hz rTMS resulted in significant improvements across various cognitive domains, including executive functioning and memory among patients with depression (Mogg et al. 2007).
In addition to focusing on psychiatric applications, an increasing number of studies are now addressing the potential therapeutic effects of rTMS in the context of cognitive neurorehabilitation (Miniussi and Rossini 2011; Stuss 2011). Indeed, 10 Hz rTMS was associated with a significant improvement in executive functioning among patients with cerebrovascular disease (Rektorova et al. 2005). Problematically, depression, schizophrenia, and cerebrovascular disease are associated with illness-driven state-based cognitive difficulties, for example, driven through neuropsychological processes, such as low mood, impaired attention, and concentration. Thus, rTMS-induced improvements in cognition may be—at least partially—through ameliorating individuals’ mental states rather than primarily enhancing cognition.
Aim
To date, the majority of studies investigating the effects of rTMS on cognitive functioning have used high-frequency stimulation, though this might be an artefact of fast rTMS being the most common paradigm, particularly in depression. A systematic review found that, in most studies, high-frequency rTMS had no significant effect on cognition (Guse et al. 2010). There was, however, variation: several studies reported improvements and three studies deterioration in cognitive functioning. Further studies have demonstrated no effect of high-frequency rTMS on working memory (Guse et al. 2013) and verbal and figural fluency (Schaller et al. 2013) in healthy patients. However, Guse et al. (2013) suggest a role for high-frequency rTMS in cognitive neuroprotection from the loss of working memory in schizophrenia. To date, there has been no systematic review of the effects of low-frequency rTMS on cognitive functioning despite low-frequency rTMS remaining a common clinical paradigm, particularly in psychosis. This paper aimed to systematically review the literature for the effects of slow (<1 Hz) rTMS in cognition.
Method
Eligibility criteria
These were defined a priori using the PICOS components (participants, interventions, comparators, outcomes, and study design) as defined by the PRISMA statement on systematic reviews (Liberati et al. 2009).
Participants Subjects without pervasive developmental disorders and neurodegenerative diseases; with any psychiatric disorder, neurological condition; and healthy were considered for inclusion. No restrictions regarding age or other population characteristics were applied.
Interventions Only studies using 1 Hz rTMS, which is utilised by the majority of low-frequency rTMS studies, were considered. No restrictions regarding other rTMS parameters were applied. Studies with online rTMS paradigms designed to induce virtual lesions were excluded, as these typically evaluate very specific neurocognitive subdomains, and their generalisability to cognitive functioning in the wider clinical populations is challengeable; and studies with 1 Hz rTMS administered in combination with other frequencies were also excluded.
Comparators Both active (e.g., high-frequency rTMS) and placebo (e.g., sham rTMS) interventions were considered. No restrictions were applied.
Outcomes Studies with one or more objective assessments of cognitive functioning were considered. No further restrictions were applied.
Study design Only randomized trials were considered for inclusion.
Literature search
Four separate electronic searches were performed using Medline (Ovid), Embase (Ovid), PsycInfo (Ovid), and the Cochrane Library as databases. Databases were last searched in September 2014. The following search terms were used: repetitive transcranial magnetic stimulation, rTMS, cognition, neurocognitive, neuropsychological, attention, reaction time, executive function, memory, learning, and processing speed. The search limits applied were English language, publication years from 1992 until 2014 and randomized trials.
Study selection
All records obtained from the electronic searches were sequentially screened on the basis of title and abstract: those that clearly did not meet the eligibility criteria were excluded, and duplicates were removed. The full texts were examined by two of the authors (C.L. and D.K.T.) for the remaining studies.
Data extraction
A data extraction form was developed based on the guidelines of the Cochrane Collaboration (Green 2011). The form was piloted on half of the included studies and revised accordingly. The following data were extracted from each: source, study design, total number of participants, sex, age, diagnosis, medication, location (of administered rTMS), number of sessions per week, frequency, coil type, total number of pulses per session, train duration and inter-train interval, intensity, comparator/control group, outcomes, and results.
Risk of bias assessment
The Cochrane Collaboration’s bias assessment software was used to measure the validity of each included study (Green 2011).
Data analysis
Due to overall heterogeneity of participants, rTMS parameters, comparator groups, and cognitive measurements, statistical combination of results for meta-analytical comparison was not considered valid. A narrative synthesis was deemed the most suitable method of data analysis. The following elements were addressed: design paradigm, neurocognitive effects, and risk of bias. Studies were organised by clinical groups namely: mood disorders, psychotic disorders, and stroke, with a fourth group, including all other single studies (epilepsy, OCD, tinnitus, and healthy participants). Full comparison of individual studies, including study size, design, parameters, and cognitive measures, used are available in the supplementary material.
Results
Search and selection of studies
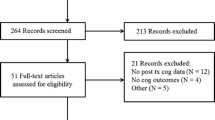
497 records were initially obtained, and 308 excluded, because of evident lack of relevance to the eligibility criteria. 90 duplicates were removed, and the eligibility of the remaining 99 studies was further assessed: 41 studies were excluded, because they did not meet the intervention criteria; 2 did not meet comparator criteria; 30 did not meet outcome criteria; 5 did not meet the study design criteria; and 1 study was excluded, because of overlap of patient data with one of the included studies. This initial search yielded 20 studies (Fig. 1).
Characteristics of included studies
Fifteen out of the 20 included studies were randomized, double-blind, sham-controlled studies, with two of these having a cross-over design. The remaining five studies consisted of two randomized double-blind non-sham-controlled cross-over studies; one randomized blind sham-controlled cross-over study; one randomized blind study; one blind randomized sham-controlled study; and one randomized open study (Table 1). Overall, the studies had small sample sizes, with the lowest number being four participants and the highest 60 (mean 29.95, SD 19.13). The participants’ ages ranged from 16 to 79 (not all studies reported a mean). Eight studies included patients with mood disorders; five included patients with psychotic disorders (schizophrenia and schizoaffective disorder); four studies involved stroke patients; and the remaining were individual studies of epilepsy, OCD, tinnitus, with one comprised healthy participants. The supplementary tables provide details of the extracted data.
The 1 Hz rTMS parameters differed substantially in the location of the stimulus, the number of rTMS sessions, the number of pulses per session, the motor threshold (MT), and the outcome measures. Areas to which rTMS was applied included the primary motor cortex (PMC), the left temporoparietal cortex (TPC), and the left or right dorsolateral prefrontal cortex (DLPFC).
The latter was the most frequently selected option. The total number of rTMS sessions ranged from one to 20, with ten sessions (five per week) being the mode. The intervention duration additionally varied from 1 day to 4 weeks. In 16 of the 20 included studies, the total number of pulses per session and the train duration/inter-train interval were either not reported or not sufficiently clearly reported. Amongst the remaining studies, these parameters differed substantially. The intensity of rTMS also varied across studies, however, in the majority, it ranged between 80 and 110 % of the MT. Three different comparator groups were used: high-frequency rTMS (10 or 20 Hz), sham stimulation, and, in one study, electroconvulsive therapy (ECT). 40 different tests assessing cognitive domains were used across studies.
Timing of cognitive measure
The time between rTMS and neurocognitive testing was evaluated. All 20 studies performed baseline testing prior to rTMS intervention. 18 of the 20 included studies performed cognitive testing immediately after completing the last session of rTMS. Of the other two, one (Januel et al. 2006) performed testing half way through the 4 weeks of rTMS; and one (Thiel et al. 2013) did post-treatment testing, but did not clarify when this occurred. 11 out of the 20 papers reported follow-up cognitive assessment after rTMS. The mean time from the final session to follow-up was 32.3 days (SD 29.08). The shortest time to follow-up was 3 days and the longest 105 days (15 weeks). 7 studies did not report follow-up and 2 were not clear as to the follow-up. One of the studies did not report follow-up (Januel et al. 2006); one of the studies was not clear as to follow-up (Thiel et al. 2013).
Neurocognitive effects of low-frequency rTMS
Due to the variety of outcome measures reported, the data were tabulated according to mental illness, broadly: mood disorders, psychotic disorders, cerebrovascular accident, and “other” (encompassing PTSD, OCD, epilepsy, anxiety, and tinnitus). A further categorization into neurocognitive domains assessed by the outcome measure used in each study was performed to facilitate cross-comparison. These categories were attention, executive function/working memory, learning and memory, and psychomotor speed and processing. No statistically significant improvement or deterioration was found in any one cognitive domain across the disease categories. Two papers (Fitzgerald et al. 2005; Hansen et al. 2011) reported statistically significant deterioration and in the cognitive domain of verbal fluency and retrieval. Furthermore, the majority of papers reported no significant improvement across the cognitive domains (Table 2).
Risk of bias
Studies were also marked with asterisks according to the strength of their methodology (*** = Low-bias risk, ** = Medium-bias risk, and * = High-bias risk). Bias assessment was calculated using RevMan 5.1 (Fig. 2). Categories of bias included randomization, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective outcome reporting, and others. Studies were rated as high risk, medium risk, and low risk depending on the highest risk level of any individual subcategory. Selective reporting was the most common serious source of bias in the studies (>50 % of included studies, see Fig. 2).
Discussion
This systematic review overall supports the general safety of rTMS and lack of harm to cognitive functioning (Anderson et al. 2006; Guse et al. 2010). Only two studies reported a significant deterioration (Fitzgerald et al. 2005; Hansen et al. 2011) in the cognitive domain of verbal fluency and retrieval. However, one study (Fitzgerald et al. 2009) found a significant improvement in verbal fluency after rTMS and three studies (Fregni et al. 2006b; Little et al. 2000; Speer et al. 2001) demonstrated no significant effect. The inconsistency of these results may reflect the variation in the methods and outcomes used to assess the impact of 1 Hz rTMS on cognitive functioning. In particular, over 40 different tests assessing various cognitive domains were used with large timing variations as to when subjects were assessed at follow-up. Furthermore, the risk of bias hinders the validity of results, with selective outcome reporting being of particular concern. Incomplete and inadequate outcome reporting is potentially a consequence of cognitive assessment being a secondary outcome in the majority of studies.
Technical factors
Type of coil and sham technique
Several coil factors may influence the effects of rTMS: the type of coil used, sham technique, and positioning during the trial (Lang et al. 2006). Two types of coil were used, the figure of eight coil and the circular coil. Circular coils produce a diffuse magnetic field over a large area and due to this lack of focality they are less used (Wassermann et al. 2008). The adequacy of the sham conditions can be challenged in some studies: in several, it involved placing the coil at a 45° angle away from the skull, which has been shown to still modulate cortical activity (Lisanby et al. 2001; Loo et al. 2000). For example, Lisanby et al. demonstrated that the tilt-induced voltage levels only 24 % below those of active stimulation (Lisanby et al. 2001). Furthermore, one study did not provide detail regarding the degree of tilt (McIntosh et al. 2004). Tilt may also affect blinding due to sensory differences to motor threshold (MT) assessments prior to treatment (Fregni et al. 2006c). Similarly, in those studies using a sham coil, different scalp sensations could unblind patients not naïve to rTMS (Fregni et al. 2006b). The purpose of sham conditions remains to find a protocol that mimics the cutaneous feelings of rTMS (Arana et al. 2008), but thus far, an adequate protocol has yet to be found (Rossi et al. 2001, 2009).
Coil positioning
Coil positioning varied between trials. One method involves locating the desired area of stimulation based on its spatial relationship to a functionally determined area, such as the motor cortex (Sparing et al. 2008). For example, to place the coil on the left DLPFC, five centimetres are measured anteriorly in a parasagittal plane from the location where the MT is determined (Nahas et al. 2007). However, the individual-level precision of such generic localisation can be challenged. For example, Herwig et al. found that the 5 cm standard method of locating the DLPFC was accurate in only 7 of 22 included participants (Herwig et al. 2001). Another method of coil positioning uses the electroencephalographic (EEG) international 10–20 system (Jasper 1958), relying on the location of cranial landmarks (e.g., nasion and preauricular points) with the coil placed at set distances from these landmarks (Nahas et al. 2007): once again, this technique is hindered by inter-individual morphological variation (Rusjan et al. 2010).
To improve the precision of stimulation, an increasing number of studies use neuronavigational methods to guide coil positioning e.g. (Herwig et al. 2003; Luber et al. 2008; Smith et al. 2007). Optical frameless stereotaxic systems incorporate imaging data and enable the coil to be positioned via three-dimensional navigation (Lefaucheur 2010; Sparing et al. 2008). Imaging data can be obtained on an individual basis using magnetic resonance imaging (MRI), functional MRI (fMRI) or positron emission tomography (PET), or utilising probabilistic imaging data from large data sets (Lefaucheur 2010; Sparing et al. 2008). However, despite the prima facie improvement in accuracy offered by neuronavigation, a large randomized controlled trial failed to demonstrate superior efficacy of fMRI-guided rTMS was not superior to conventionally applied rTMS or sham stimulation among patients with treatment-resistant auditory verbal hallucinations (AVH) (Slotema et al. 2011).
Stimulation protocol
Stimulus intensity is determined in relation to the MT (Nahas et al. 2007), and in the majority of studies, it ranged between 80 and 110 % of the MT. Such variation may affect the consistency of results; work on corticospinal excitability has shown, for example, that 115 % rTMS led to a reduction in motor evoked potentials (MEPs), whereas when given at 85 % of the MT, it did not (Fitzgerald et al. 2002). The choice of correct MT threshold has been relatively underexplored in clinical rTMS protocols despite evidence to suggest that both age and medication exhibit significant effects on the MT.
rTMS is known to be less effective among older participants (Figiel et al. 1998; Su et al. 2005). One explanation for this is the increased distance between scalp and cortex among older adults due to age-related cerebral atrophy, with the strength of the magnetic field drops exponentially with distance from the coil (Wassermann et al. 2008). To compensate for cerebral atrophy, the intensity of rTMS can be adjusted considering that the rate of atrophy is not symmetrical across cortical areas (Stokes et al. 2005, 2007). In a sample of depressed patients with an age range of 55–75 years with MT adjusted for distance between the scalp and the cortex, the intensity of rTMS ranged from 103 to 141 % of the MT. Out of 18 patients, four achieved remission and a further five were partial responders (Nahas et al. 2004). These results suggest that correcting for age-related atrophic changes may improve rTMS outcomes in older patients.
Medication may also affect the required rTMS stimulus intensity, and, for example, both the antidepressants citalopram (Minelli et al. 2010; Robol et al. 2004) and clomipramine (Minelli et al. 2010) have been shown to increase the necessary MT. AEDs, such as lamotrigine and phenytoin, increase the MT due to their blocking action on voltage-sensitive sodium channels (Paulus et al. 2008; Wassermann et al. 2008). Long-term use of benzodiazepines also significantly increases the MT (Palmieri et al. 1999). The majority of participants in the included studies were on psychotropic medication that may have affected cognitive functioning and cortical excitability, although this was inconsistently reported in trials, and few evaluated this as a confounder. Anti-epileptic drugs (AEDs), antipsychotics, antidepressants, and benzodiazepines all have potential adverse cognitive side effects (Drane and Meador 2002; Elie et al. 2009; Hori et al. 2012; Schachter 2007). Evaluation of drug effects can be difficult due to the considerable variation between the various drugs, individual susceptibility to side effects, and fluid changes in cognition from functional aspects of the illness itself. For any meaningful comparison to be made between studies, full reporting of study design must be undertaken.
If comparisons are to be made between studies, reporting of study design is of paramount importance. Stimulation parameters, including the number of pulses, the train duration/inter-train interval, and the number of sessions varied considerably or were not consistently recorded: 16 out of the 20 studies did not adequately report these parameters.
Age is also a confounding factor, and similarly underexplored, in relation to neurocognitive testing. In addition to age-related changes to the MT, age-related decline of performance on various cognitive tasks is well documented, e.g., (Brickman et al. 2007; Wielgos et al. 1999). The age range of participants in the included studies was 16–79 and a wide age range was used in each individual study. However, most studies did not report the mean age of their participants making it impossible to draw any conclusions.
Conclusion
In summary, no definite conclusions can be drawn at this time, regarding the effects of 1 Hz rTMS on cognitive functioning. Calling for more research is futile if that research can produce no meaningful conclusions: to date the lack of unambiguous findings is not due solely to a lack of research—though the field remains underexplored—but is far more hindered by methodological issues. Nevertheless, there are lessons to be learned regarding protocols for rTMS use, confounding factors in studies, and a theory of pre-conditioning and post-conditioning that could greatly improve the quality and applicability of rTMS in mood disorders, psychotic illness, stroke, epilepsy, and other disorders.
There are several clear areas that future research in this field will need to address. The obvious area of need is standardisation—or at least adequate reporting—across several domains: technical; rTMS protocols; and neurocognitive outcome measures. This is true of the broader neuromodulatory field, not limited to cognitive effects. Individual trials are unlikely to be sufficiently powered to elucidate all of these factors, but if they are at least appropriately reported, then bigger data set analysis of these and demographic factors will allow valid cross-comparison and meta-analytic analysis of future work.
The figure of eight coil has largely superseded the circular coil, and it is unlikely much future work will be undertaken with the latter. Blinding and sham condition paradigms remain problematically inconsistent, but despite the issue of the lack of sensation, sham coils appear a better proposition than coil tilting. With regard to coil siting, whilst we note the negative findings of Sloetma et al., it is our opinion that neuronavigation is an inherently superior paradigm as its functional approach addresses individual variation. However, further studies are warranted if this argument is to be proved or disproved.
Standardised cognitive batteries would hugely facilitate across-study comparisons and the replication of studies. The MATRICs initiative in schizophrenia studies is a worthy reference point in this regard. As with MATRICS, good test–retest reliability, practicality of test usage, relationship to functional outcome, response to pharmacological adjuncts, and use as a repeated measure should be characteristics of the included tests (Green et al. 2004). Such protocol consistency is, especially, important given that any protocol discrepancies are compounded by confounding factors intrinsic to the populations studied: in particular, age-related changes in cognition and concomitant pharmacotherapy.
Finally, it may be the case that pre-conditioning and post-conditioning of the brain are necessary to take full advantage of the positive effects of rTMS. This hypothesis of pre-conditioning the brain is already borne out through studies on the effects of pharmaceuticals in rTMS studies (Fregni et al. 2006a), in which the effects of 1 Hz rTMS depended on the state of cortical excitability at the time of stimulation.
In addition to pharmaceutical pre-conditioning, studies have used transcranial direct current stimulation (tDCS) to modulate the effects of 1 Hz rTMS. Siebner et al. demonstrated that excitatory anodal tDCS caused 1 Hz rTMS to further reduce cortical excitability, whereas inhibitory cathodal tDCS led to an increase in excitability following 1 Hz rTMS (Siebner et al. 2004). Similarly, Iyer et al. found that 6 Hz rTMS enhanced the inhibitory effects of 1 Hz rTMS (Iyer et al. 2003). The same pattern of results was also obtained by Lang et al., in which the direction of 5 Hz rTMS was determined by preceding tDCS conditioning of motor cortex excitability (Lang et al. 2004). These findings have potentially important implications for the included studies with a cross-over design, e.g., (Fitzgerald et al. 2009; Little et al. 2000). Since some of these studies crossed-over patients from low-frequency rTMS to high-frequency rTMS and vice versa, it is possible that carry-over effects confounded the results, but also that cross-over studies may compound positive effects.
A further point in this regard is that there is reasonably good basic neuroscience data that rTMS can enhance neuronal plasticity—the mechanism, indeed, that would underlie any putative cognitive enhancement. However, with this in mind, there is an obvious dearth of utilising parallel cognitive remediation during the trial rTMS period.
rTMS has been in existence since the 1980s. Despite this, and the ongoing interest in its potential clinical roles, it remains incompletely understood. There are data to support its utility in several neuropsychiatric disorders and, somewhat more speculatively, to enhance cognition. At this time, it remains unclear how much the somewhat ambivalent data represent the technique’s fundamental limitations, and how much the numerous confounders are clouding any underlying improvement. If practical aspects mean that smaller study size remain the norm, this should at least be done within the context of standardised reporting that will allow work to fit within bigger subsequent data sets.
Change history
21 June 2019
The original version of this article unfortunately contained a mistake. The author would like to include the below acknowledgement section.
21 June 2019
The original version of this article unfortunately contained a mistake. The author would like to include the below acknowledgement section.
References
Anderson B, Mishory A, Nahas Z, Borckardt JJ, Yamanaka K, Rastogi K, George MS (2006) Tolerability and safety of high daily doses of repetitive transcranial magnetic stimulation in healthy young men. J ECT 22:49–53
Arana AB et al (2008) Focal electrical stimulation as a sham control for repetitive transcranial magnetic stimulation: Does it truly mimic the cutaneous sensation and pain of active prefrontal repetitive transcranial magnetic stimulation? Brain Stimul 1:44–51
Aydin-Abidin S, Trippe J, Funke K, Eysel UT, Benali A (2008) High-and low-frequency repetitive transcranial magnetic stimulation differentially activates c-Fos and zif268 protein expression in the rat brain. Exp Brain Res 188:249–261
Berlim MT, Neufeld NH, Van den Eynde F (2013) Repetitive transcranial magnetic stimulation (rTMS) for obsessive-compulsive disorder (OCD): an exploratory meta-analysis of randomized and sham-controlled trials. J Psychiatr Res 47:999–1006
Brickman AM, Habeck C, Zarahn E, Flynn J, Stern Y (2007) Structural MRI covariance patterns associated with normal aging and neuropsychological functioning. Neurobiol Aging 28:284–295
Demirtas-Tatlidede A, Vahabzadeh-Hagh AM, Pascual-Leone A (2013) Can noninvasive brain stimulation enhance cognition in neuropsychiatric disorders? Neuropharmacology 64:566–578
Doi W, Sato D, Fukuzako H, Takigawa M (2001) c-Fos expression in rat brain after repetitive transcranial magnetic stimulation. Neuroreport 12:1307–1310
Drane DL, Meador KJ (2002) Cognitive and behavioral effects of antiepileptic drugs. Epilepsy Behav 3:49–53
Elie D, Poirier M, Chianetta J, Durand M, Gregoire C, Grignon S (2009) Cognitive effects of antipsychotic dosage and polypharmacy: a study with the BACS in patients with schizophrenia and schizoaffective disorder. J Psychopharmacol
Figiel GS, Epstein C, McDonald WM, Amazon-Leece J, Figiel L, Saldivia A, Glover S (1998) The use of rapid-rate transcranial magnetic stimulation (rTMS) in refractory depressed patients. J Neuropsychiatr Clin Neurosci 10:20–25
Fitzgerald PB, Brown TL, Daskalakis ZJ, Chen R, Kulkarni J (2002) Intensity-dependent effects of 1 Hz rTMS on human corticospinal excitability. Clin Neurophysiol 113:1136–1141
Fitzgerald PB, Benitez J, Daskalakis JZ, Brown TL, Marston NA, De Castella A, Kulkarni J (2005) A double-blind sham-controlled trial of repetitive transcranial magnetic stimulation in the treatment of refractory auditory hallucinations. J Clin Psychopharmacol 25:358–362
Fitzgerald PB, Hoy K, Daskalakis ZJ, Kulkarni J (2009) A randomized trial of the anti-depressant effects of low- and high-frequency transcranial magnetic stimulation in treatment-resistant depression. Depression Anxiety 26:229–234
Fregni F et al (2006a) Homeostatic effects of plasma valproate levels on corticospinal excitability changes induced by 1 Hz rTMS in patients with juvenile myoclonic epilepsy. Clin Neurophysiol 117:1217–1227
Fregni F et al (2006b) A sham-controlled trial of a 5-day course of repetitive transcranial magnetic stimulation of the unaffected hemisphere in stroke patients. Stroke 37:2115–2122
Fregni F et al (2006c) A randomized clinical trial of repetitive transcranial magnetic stimulation in patients with refractory epilepsy. Ann Neurol 60:447–455
Gough PM, Nobre AC, Devlin JT (2005) Dissociating linguistic processes in the left inferior frontal cortex with transcranial magnetic stimulation. J Neurosci 25:8010–8016
Green S (2011) Cochrane handbook for systematic reviews of interventions version 5.1. 0 [updated March 2011] The Cochrane Collaboration
Green MF, Kern RS, Heaton RK (2004) Longitudinal studies of cognition and functional outcome in schizophrenia: implications for MATRICS. Schizophr Res 72:41–51
Greenberg BD et al (1997) Effect of prefrontal repetitive transcranial magnetic stimulation in obsessive-compulsive disorder: a preliminary study. Am J Psychiatry 154:867–869
Guse B, Falkai P, Wobrock T (2010) Cognitive effects of high-frequency repetitive transcranial magnetic stimulation: a systematic review. J Neural Transm 117:105–122
Guse B, Falkai P, Gruber O, Whalley H, Gibson L, Hasan A, Obst K, Dechent P, McIntosh A, Suchan B, Wobrock T (2013) The effect of long-term high frequency repetitive transcranial magnetic stimulation on working memory in schizophrenia and healthy controls - a randomized placebo controlled, double-blind fMRI study. Behav Brain Res 237:300–307
Hansen PEB et al (2011) Low-frequency repetitive transcranial magnetic stimulation inferior to electroconvulsive therapy in treating depression. J ECT 27:26–32
Herwig U, Padberg F, Unger J, Spitzer M, Schonfeldt-Lecuona C (2001) Transcranial magnetic stimulation in therapy studies: examination of the reliability of Äústandard, Äù coil positioning by neuronavigation. Biol Psychiatry 50:58–61
Herwig U, Lampe Y, Juengling FD, Wunderlich A, Walter H, Spitzer M, Schönfeldt-Lecuona C (2003) Add-on rTMS for treatment of depression: a pilot study using stereotaxic coil-navigation according to PET data. J Psychiatr Res 37:267–275
Hoffman RE et al (2005) Temporoparietal transcranial magnetic stimulation for auditory hallucinations: safety, efficacy and moderators in a fifty patient sample. Biol Psychiatry 58:97–104
Hoffman RE, Wu K, Pittman B, Cahill JD, Hawkins KA, Fernandez T, Hannestad J (2013) Transcranial magnetic stimulation of Wernicke, Äôs and right homologous sites to curtail, Äúvoices, Äù: a randomized trial. Biol Psychiatr 73:1008–1014
Hoogendam JM, Ramakers GM, Di Lazzaro V (2010) Physiology of repetitive transcranial magnetic stimulation of the human brain Brain stimulation 3:95–118
Hoppner J, Schulz M, Irmisch G, Mau R, Schlufke D, Richter J (2003) Antidepressant efficacy of two different rTMS procedures. Eur Arch Psychiatry Clin Neurosci 253:103–109
Hori H, Yoshimura R, Katsuki A, Hayashi K, Ikenouchi-Sugita A, Umene-Nakano W, Nakamura J (2012) The cognitive profile of aripiprazole differs from that of other atypical antipsychotics in schizophrenia patients. J Psychiatr Res 46:757–761
Hovington CL, McGirr A, Lepage M, Berlim MT (2013) Repetitive transcranial magnetic stimulation (rTMS) for treating major depression and schizophrenia: a systematic review of recent meta-analyses. Ann Med 45:308–321. doi:10.3109/07853890.2013.783993
Hulme SR, Jones OD, Abraham WC (2013) Emerging roles of metaplasticity in behaviour and disease. Trends Neurosci 36:353–362
Iyer MB, Schleper N, Wassermann EM (2003) Priming stimulation enhances the depressant effect of low-frequency repetitive transcranial magnetic stimulation. J Neurosci 23:10867–10872
Januel D et al (2006) A double-blind sham controlled study of right prefrontal repetitive transcranial magnetic stimulation (rTMS): therapeutic and cognitive effect in medication free unipolar depression during 4 weeks. Prog Neuropsychopharmacol Biol Psychiatry 30:126–130
Jasper HH (1958) The ten twenty electrode system of the international federation. Electroencephalogr Clin Neurophysiol 10:371–375
Kang JI, Kim C-H, Namkoong K, Lee CI, Kim SJ (2009) A randomized controlled study of sequentially applied repetitive transcranial magnetic stimulation in obsessive-compulsive disorder. J Clin Psychiatr 70:1645–1651
Khedr E, Abdel-Fadeil M, Farghali A, Qaid M (2009) Role of 1 and 3 Hz repetitive transcranial magnetic stimulation on motor function recovery after acute ischaemic stroke. Eur J Neurol 16:1323–1330
Khedr E, Abo-Elfetoh N, Rothwell J, El-Atar A, Sayed E, Khalifa H (2010) Contralateral versus ipsilateral rTMS of temporoparietal cortex for the treatment of chronic unilateral tinnitus: comparative study. Eur J Neurol 17:976–983
Kim Y-H et al (2006) Repetitive transcranial magnetic stimulation-induced corticomotor excitability and associated motor skill acquisition in chronic stroke. Stroke 37:1471–1476
Kim BR, Kim D-Y, Chun MH, Yi JH, Kwon JS (2010) Effect of repetitive transcranial magnetic stimulation on cognition and mood in stroke patients: a double-blind, sham-controlled trial. Am J Phys Med Rehabil 89:362–368
Kleinjung T et al (2005) Long-term effects of repetitive transcranial magnetic stimulation (rTMS) in patients with chronic tinnitus. Otolaryngol Head Neck Surg 132:566–569
Koren D, Shefer O, Chistyakov A, Kaplan B, Feinsod M, Klein E (2001) Neuropsychological effects of prefrontal slow rTMS in normal volunteers: a double-blind sham-controlled study. J Clin Exp Neuropsychol 23:424–430
Landgrebe M et al (2013) P 111. Repetitive transcranial magnetstimulation (rTMS) for the treatment of chronic tinnitus: results of the german multicenter study. Clin Neurophysiol 124:e117–e118
Lang N, Siebner HR, Ernst D, Nitsche MA, Paulus W, Lemon RN, Rothwell JC (2004) Preconditioning with transcranial direct current stimulation sensitizes the motor cortex to rapid-rate transcranial magnetic stimulation and controls the direction of after-effects. Biol Psychiatry 56:634–639
Lang N, Harms J, Weyh T, Lemon RN, Paulus W, Rothwell JC, Siebner HR (2006) Stimulus intensity and coil characteristics influence the efficacy of rTMS to suppress cortical excitability. Clin Neurophysiol 117:2292–2301
Langguth B, Eichhammer P, Wiegand R, Marienhegen J, Maenner P, Jacob P, Hajak G (2003) Neuronavigated rTMS in a patient with chronic tinnitus. Effects of 4 weeks treatment. NeuroReport 14:980–997
Lefaucheur J-P (2010) Why image-guided navigation becomes essential in the practice of transcranial magnetic stimulation. Clin Neurophysiol 40:1–5
Liberati A et al (2009) The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Ann Intern Med 151:W-65–W-94
Lisanby SH, Gutman D, Luber B, Schroeder C, Sackeim HA (2001) Sham TMS: intracerebral measurement of the induced electrical field and the induction of motor-evoked potentials. Biol Psychiatry 49:460–463
Little JT et al (2000) Cognitive effects of 1-and 20-hertz repetitive transcranial magnetic stimulation in depression: preliminary report. Cognit Behav Neurol 13:119
Loo CK, Taylor JL, Gandevia SC, McDarmont BN, Mitchell PB, Sachdev PS (2000) Transcranial magnetic stimulation (TMS) in controlled treatment studies: are some, Äúsham, Äù forms active? Biol Psychiatry 47:325–331
Luber B et al (2008) Remediation of sleep-deprivation-induced working memory impairment with fMRI-guided transcranial magnetic stimulation. Cereb Cortex 18:2077–2085
Mantovani A, Simpson HB, Fallon BA, Rossi S, Lisanby SH (2010) Randomized sham-controlled trial of repetitive transcranial magnetic stimulation in treatment-resistant obsessive-compulsive disorder. Int J Neuropsychopharmacol 13:217–227
Martis B, Alam D, Dowd SM, Hill SK, Sharma RP, Rosen C, Pliskin N, Martin E, Carson V, Janicak PG (2003) Neurocognitive effects of repetitive transcranial magnetic stimulation in severe depression. Clin Neurophysiol 114(6):1125–1132
McIntosh AM, Semple D, Tasker K, Harrison LK, Owens DG, Johnstone EC, Ebmeier KP (2004) Transcranial magnetic stimulation for auditory hallucinations in schizophrenia. Psychiatry Res 127:9–17
Minelli A, Bortolomasi M, Scassellati C, Salvoro B, Avesani M, Manganotti P (2010) Effects of intravenous antidepressant drugs on the excitability of human motor cortex: a study with paired magnetic stimulation on depressed patients. Brain Stimul 3:15–21
Miniussi C, Rossini PM (2011) Transcranial magnetic stimulation in cognitive rehabilitation. Neuropsychol Rehabilitat 21:579–601
Miniussi C, Ruzzoli M, Walsh V (2010) The mechanism of transcranial magnetic stimulation in cognition. Cortex 46:128–130
Mogg A et al (2007) Repetitive transcranial magnetic stimulation for negative symptoms of schizophrenia: a randomized controlled pilot study. Schizophr Res 93:221–228
Nahas Z et al (2004) Safety and benefits of distance-adjusted prefrontal transcranial magnetic stimulation in depressed patients 55–75 years of age: a pilot study. Depress Anxiety 19:249–256
Nahas Z et al (2007) Methods of administering transcranial magnetic stimulation. In: George MS, Belmaker RH (eds) Transcranial magnetic stimulation in clinical psychiatry. American Psychiatric Publishing Inc, Washington DC, pp 39–59
Palmieri MG, Iani C, Scalise A, Desiato MT, Loberti M, Telera S, Caramia MD (1999) The effect of benzodiazepines and flumazenil on motor cortical excitability in the human brain. Brain Res 815:192–199
Paulus W et al (2008) State of the art: pharmacologic effects on cortical excitability measures tested by transcranial magnetic stimulation Brain stimulation 1:151–163
Pell GS, Roth Y, Zangen A (2011) Modulation of cortical excitability induced by repetitive transcranial magnetic stimulation: influence of timing and geometrical parameters and underlying mechanisms. Prog Neurobiol 93:59–98
Rektorova I, Megova S, Bares M, Rektor I (2005) Cognitive functioning after repetitive transcranial magnetic stimulation in patients with cerebrovascular disease without dementia: a pilot study of seven patients. J Neurol Sci 229:157–161
Ridding MC, Rothwell JC (2007) Is there a future for therapeutic use of transcranial magnetic stimulation? Nat Rev Neurosci 8:559–567
Robol E, Fiaschi A, Manganotti P (2004) Effects of citalopram on the excitability of the human motor cortex: a paired magnetic stimulation study. J Neurol Sci 221:41–46
Rossi S et al (2001) Prefontal cortex in long-term memory: an interference approach using magnetic stimulation. Nat Neurosci 4:948–952
Rossi S, Hallett M, Rossini PM, Pascual-Leone A (2009) Safety, ethical considerations, and application guidelines for the use of transcranial magnetic stimulation in clinical practice and research. Clin Neurophysiol 120:2008–2039
Rusjan PM, Barr MS, Farzan F, Arenovich T, Maller JJ, Fitzgerald PB, Daskalakis ZJ (2010) Optimal transcranial magnetic stimulation coil placement for targeting the dorsolateral prefrontal cortex using novel magnetic resonance image-guided neuronavigation. Hum Brain Mapp 31:1643–1652
Schachter SC (2007) Currently available antiepileptic drugs. Neurotherapeutics 4:4–11
Schaller G, Lenz B, Friedrich K, Dygon D, Richter-Schmidinger T, Sperling W, Kornhuber J (2013) No evidence for effects of a high-frequency repetitive transcranial stimulation series on verbal and figural fluency and TAP task performance in healthy male volunteers. Neuropsychobiology 67(2):69–73
Schneider AL, Schneider TL, Stark H (2008) Repetitive transcranial magnetic stimulation (rTMS) as an augmentation treatment for the negative symptoms of schizophrenia: a 4-week randomized placebo controlled study. Brain Stimul 1:106–111
Schulze-Rauschenbach SC, Harms U, Schlaepfer TE, Maier W, Falkai P, Wagner M (2005) Distinctive neurocognitive effects of repetitive transcranial magnetic stimulation and electroconvulsive therapy in major depression. Br J Psychiatry 186:410–416
Siebner HR, Lang N, Rizzo V, Nitsche MA, Paulus W, Lemon RN, Rothwell JC (2004) Preconditioning of low-frequency repetitive transcranial magnetic stimulation with transcranial direct current stimulation: evidence for homeostatic plasticity in the human motor cortex. J Neurosci 24:3379–3385
Slotema CW et al (2011) Can low-frequency repetitive transcranial magnetic stimulation really relieve medication-resistant auditory verbal hallucinations? Negative results from a large randomized controlled trial. Biol Psychiatry 69:450–456
Smith JA, Mennemeier M, Bartel T, Chelette KC, Kimbrell T, Triggs W, Dornhoffer JL (2007) Repetitive transcranial magnetic stimulation for tinnitus: a pilot study. Laryngoscope 117:529–534
Sparing R, Buelte D, Meister IG, Paus T, Fink GR (2008) Transcranial magnetic stimulation and the challenge of coil placement: a comparison of conventional and stereotaxic neuronavigational strategies. Hum Brain Mapp 29:82–96
Speer AM, Repella JD, Figueras S, Demian NK, Kimbrell TA, Wasserman EM, Post RM (2001) Lack of adverse cognitive effects of 1 Hz and 20 Hz repetitive transcranial magnetic stimulation at 100% of motor threshold over left prefrontal cortex in depression. J ECT 17:259–263
Stokes MG, Chambers CD, Gould IC, Henderson TR, Janko NE, Allen NB, Mattingley JB (2005) Simple metric for scaling motor threshold based on scalp-cortex distance: application to studies using transcranial magnetic stimulation. J Neurophysiol 94:4520–4527
Stokes MG, Chambers CD, Gould IC, English T, McNaught E, McDonald O, Mattingley JB (2007) Distance-adjusted motor threshold for transcranial magnetic stimulation. Clin Neurophysiol 118:1617–1625
Stuss DT (2011) The future of cognitive neurorehabilitation. Neuropsychol Rehabilitat 21:755–768
Su T-P, Huang C-C, Wei I-H (2005) Add-on rTMS for medication-resistant depression: a randomized, double-blind, sham-controlled trial in Chinese patients. J Clin Psychiatr 66:930–937
Takeuchi N, Tada T, Toshima M, Chuma T, Matsuo Y, Ikoma K (2008) Inhibition of the unaffected motor cortex by 1 Hz repetitive transcranial magnetic stimulation enhances motor performance and training effect of the paretic hand in patients with chronic stroke. J Rehabil Med 40:298–303
Thiel A et al (2013) Effects of noninvasive brain stimulation on language networks and recovery in early poststroke aphasia. Stroke 44:2240–2246
Tracy DK, David AS (2015) Clinical neuromodulation in psychiatry: the state of the art or an art in a state? Current evidence and future challenges. BJPsych Advances 21:396–404
Tracy DK et al (2011) It’s not what you say but the way that you say it: an fMRI study of differential lexical and non-lexical prosodic pitch processing. BMC Neurosci 12:128
Tracy DK, de Sousa de Abreu M, Nalesnik N, Mao L, Lage C, Shergill SS (2014). Neuroimaging effects of 1Hz right temporo-parietal rTMS on normal auditory processing: implications for clinical hallucination treatment paradigms. J Clin Neurophys 31(6):541–546
Tracy DK, Shergill SS, David AS, Fonagy P, Zaman R, Downar J, Eliott E, Bhui K (2015). Self-harm and suicidal acts: a suitable case for treatment of impulsivity-driven behaviour with repetitive transcranial magnetic stimulation (rTMS). BJPsych Open 1(1):87–91
Van den Eynde F et al (2010) Repetitive transcranial magnetic stimulation reduces cue-induced food craving in bulimic disorders. Biol Psychiatry 67:793–795
Van den Eynde F, Guillaume S, Broadbent H, Campbell I, Schmidt U (2013) Repetitive transcranial magnetic stimulation in anorexia nervosa: a pilot study. Eur Psychiatry 28:98–101
Waldowski K, Seniew J, Leoniak M, Iwanski S, Czonkowska A (2012) Effect of low-frequency repetitive transcranial magnetic stimulation on naming abilities in early-stroke aphasic patients: a prospective, randomized, double-blind sham-controlled study. Sci World J
Wassermann E, Epstein C, Ziemann U (2008) Oxford handbook of transcranial stimulation. Oxford University Press
Watts BV, Landon B, Groft A, Young-Xu Y (2012) A sham controlled study of repetitive transcranial magnetic stimulation for posttraumatic stress disorder. Brain Stimul 5:38–43
Wielgos M, Cunningham WR, Cynthia (1999) Age-related slowing on the digit symbol task: longitudinal and cross-sectional analyses. Exp Aging Res 25:109–120
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no financial or other conflict of interest.
Funding
No funding or financial support was provided for the conduct of this research.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Lage, C., Wiles, K., Shergill, S.S. et al. A systematic review of the effects of low-frequency repetitive transcranial magnetic stimulation on cognition. J Neural Transm 123, 1479–1490 (2016). https://doi.org/10.1007/s00702-016-1592-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00702-016-1592-8