Abstract
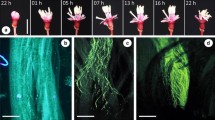
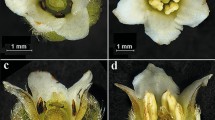
Paullinia weinmanniifolia (Sapindaceae) is an endemic Brazilian climber found in the Atlantic Forest. The flowers are arranged in synflorescences with approximately 34 thyrses. In the studied material, each thyrse produced 85.8 male flowers and 15.8 female flowers. The small, diclinous and zygomorphic flowers present a sophisticated morphology, described here in detail. They are visited by many insects, with bees being the main pollinators. Sex expression was investigated by monitoring one population of restinga in Maricá Environmental Protection Area, Rio de Janeiro State, Brazil, during 2–4 years. Experiments were conducted taking into account thyrse, synflorescence, plant and population. Most individuals showed a duodichogamic sequence of flowering (male–female–male). However, the population overall had a more complex flowering pattern: some individuals were protogynous, others protandrous; a few individuals produced flowers of only one sex, and some individuals changed sex expression in the second year of the study, either from male to female or from female to male. This was the first ever labile sex expression recorded for the tribe Paullinieae. Anthetic female and male flowers were never found simultaneously in the same thyrse or synflorescence. Nevertheless, the two flower morphs overlapped, though rarely, within the same individual. The pattern of flowering observed in this species maximizes the level of outcrossing, since the temporal separation of male and female flowers on the same plant is precise enough for the species to be regarded as (obligatorily) xenogamous.






Similar content being viewed by others
References
Acevedo-Rodríguez P (1993) Systematics of Serjania (Sapindaceae). Part I: a revision of Serjania sect. Platycoccus. Mem New York Bot Gard 67:1–93
Acevedo-Rodríguez P, van Welzen PC, Adema F, van der Ham RWJM (2010) Sapindaceae. In: Kubitzki K (ed) Flowering plants, Eudicots: Sapindales, Cucurbitales, Myrtaceae. The families and genera of vascular plants, vol 10. Springer, Berlin, pp 357–407
Aluri JSR, Subba Reddi C, Rama Das K (1998) Temporal dioecism and pollination by wasps and bees in Allophylus serratus (Roxb.) Radlk. (Sapindaceae). Pl Spec Biol 13:1–5
Appanah S (1982) Pollination of androdioecious Xerospermum intermedium Radlk. (Sapindaceae) in a rain forest. Biol J Linn Soc 18:11–34
Barrett SCH (2002) The evolution of plant sexual diversity. Nat Rev Genet 3:274–284
Bawa KS (1977) The reproductive biology of Cupania guatemalensis Radlk. (Sapindaceae). Evolution 31:52–63
Buerki S, Forest F, Acevedo-Rodríguez P, Callmander MW, Nylander JAA, Harrington M, Sanmartín I, Küpfer P, Alvarez N (2009) Plastid and nuclear DNA markers reveal intricate relationships at subfamilial and tribal levels in the soapberry family (Sapindaceae). Molec Phylogen Evol 51:238–258
Cane JH (1993) Reproductive role of sterile pollen in cryptically dioecious species of flowering plants. Curr Sci 65:223–225
Charlesworth D, Charlesworth B (1978) A model for the evolution of dioecy and gynodioecy. Amer Naturalist 112:975–997
Croat TB (1976) Sapindaceae. In: Flora of Panama. Ann Missouri Bot Gard 63:509–522
De Jong PC (1976) Flowering and sex expression in Acer L. A biosystematic study. Meded Landbouwhogeschool Wageningen 76:1–201
Endress PK, Matthews ML (2006) Elaborate petals and staminodes in eudicots: diversity, function, and evolution. Org Divers Evol 6:257–293
Escobar JR, Correa MPF, Aguilera FJP (1984) Estruturas florais, floração e técnicas para a polinização controlada do guaranazeiro. Pesq Agropec Brasil 19:615–622
Ferrucci MS (1991) Sapindaceae. In: Spichiger R, Ramella L (eds) Flora del Paraguay. Conservatoire et Jardin Botaniques de la Ville de Genève and Missouri Botanical Garden, pp 1–144
Fournier LA (1974) Un método cuantitativo para la medición de características fenológicas en árboles. Turrialba 24:422–423
Gadek PA, Fernando ES, Quinn CJ, Hoot SB, Terrazas T, Sheahan MC, Chase MW (1996) Sapindales: molecular delimitations and infraordinal groups. Amer J Bot 83:802–811
Golenberg EM, West NH (2013) Hormonal interactions and gene regulation can link monoecy and environmental plasticity to the evolution of dioecy in plants. Amer J Bot 100:1–16
Harrington MG, Edwards KJ, Johnson SA, Chase MW, Gadek PA (2005) Phylogenetic inference in Sapindaceae sensu lato using plastid matK and rbcL DNA sequences. Syst Bot 30:366–382
Johansen DA (1940) Plant microtechnique. McGraw-Hill Book Company, New York
Judd WS, Campbell CS, Kellogg EA, Stevens PF, Donoghue MJ (2009) Sistemática Vegetal: um enfoque filogenético, 3rd edn. Artmed, Porto Alegre, pp 438–440
Kearns CA, Inouye D (1993) Techniques for pollinations biologists. University Press of Colorado, Boulder
Korpelainen H (1998) Labile sex expression in plants. Biol Rev Cambridge Philos Soc 73:157–180
Lloyd DG, Webb CJ (1986) The avoidance of interference between the presentation of pollen and stigmas in Angiosperms I. Dichogamy. New Zealand J Bot 24:135–162
Luo S, Zhang D, Renner S (2007) Duodichogamy and androdioecy in the Chinese Phyllanthaceae Bridelia tomentosa. Amer J Bot 94:260–265
Menezes LFT, Araújo DSD (2005) Formações vegetais da restinga da Marambaia, Rio de Janeiro. In: Menezes LFT, Peixoto AL, Araujo DSD (eds) História Natural da Marambaia. EDUR, Seropédica, pp 67–120
Moreira Filho A, Ribeiro OC, Ferreira MA, Martins GA (1975) Polinização e polinizadores de guaraná. Inf Téc ACAR-AM 3:4–7
Ormond WT, Pinheiro MCB, Lima HA, Correia MCR, Castro AC (1991) Sexualidade das plantas da restinga de Maricá, RJ. Bol Mus Nac Rio de Janeiro Bot 87:1–24
Paula NMC (1989) Aspectos da biologia reprodutiva de Paullinia rugosa Benth. ex Radlk. (Sapindaceae). Dissertation. Instituto Nacional de Pesquisa da Amazônia (INPA) e Fundação Universidade do Amazonas
Radford AE, Dickison WC, Massey JR, Bell CR (1974) Vascular plant systematics. Harper and Row Publishers, New York
Radlkofer LAT (1931–1934) Sapindaceae. In: Engler A (ed) Das Pflanzenreich IV, 165 (Heft 98 a–h). Verlag von Wilhelm Engelmann, Leipzig, pp 1–1539
Rama Das K, Ruddi CS, Aluri RJS, Atluri JB (1997) Sexual system and pollination ecology of Cardiospermum halicacabum L. (Sapindaceae). J Bombay Nat Hist Soc 94:333–341
Renner SS (2001) How common is heterodichogamy? Trends Ecol Evol 16:595–597
Renner SS, Beenken L, Grimm GW, Kocyan A, Ricklefs RE (2007) The evolution of dioecy, heterodichogamy, and labile sex expression in Acer. Evolution 61–11:2701–2719
Robbertse PJ, Du Toit ES, Cloete MO (2011) Gender expression and inflorescence structure of Pappea capensis Eckl. and Zeyh. (Sapindaceae). S African J Bot 77:425–429
Sato T (2002) Phenology of sex expression and gender variation in a heterodichogamous maple, Acer japonicum. Ecology 83:1226–1238
Shang H, Luo Y-B, Bai W-N (2012) Influence of asymmetrical mating patterns and male reproductive success on the maintenance of sexual polymorphism in Acer pictum subsp. mono (Aceraceae). Molec Ecol 21:3869–3878
StatSoft Inc (2007) Statistica (data analysis software system) version 8.0. http://www.statsoft.com
Subba Reddi C, Reddi EUB, Reddi NS, Reddi PS (1983) Reproductive ecology of Sapindus emarginatus Vahl (Sapindaceae). Proc Indian Acad Sci Pl Sci 49:57–72
Vamosi JC, Otto SP, Barrett SCH (2003) Phylogenetic analysis of the ecological correlates of dioecy. J Evol Biol 16:1006–1018
Vary LB, Sakai AK, Weller SG (2011) Morphological and functional sex expression in the Malagasy endemic Tina striata (Sapindaceae). Amer J Bot 98(6):1040–1048
Verdú M, Gleiser G (2005) Adaptative evolution of reproductive and vegetative traits driven by breeding systems. New Phytol 169:409–417
Vogel S (1990) The role of scent glands in pollination: On the structure and function of osmophores. Amerind Publishing Co., Pvt. Ltd, New Delhi, New Delhi
Zapata TR, Arroyo MTK (1978) Plant reproductive ecology of a secondary deciduous tropical forest in Venezuela. Biotropica 10:221–230
Acknowledgments
We would like to thank the entomologists Dr. Antonio José Mayhé Nunes, from the Universidade Federal Rural do Rio de Janeiro (UFRRJ), Dra. Cátia Mello Patiu, Dra. Márcia Souto Couri, and biologist Alexandre Soares, from Museu Nacional, Universidade Federal do Rio de Janeiro (UFRJ), for identification of the insects, Mrs. Glória Gonçalves, for the illustrations, Dr. Ivo Abraão Araújo da Silva, for support on statistical aspects, and Dra. Cristine Rodrigues Benevides, for criticisms and suggestions.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None.
Additional information
Handling editor: Peter K. Endress.
Rights and permissions
About this article
Cite this article
de Lima, H.A., Somner, G.V. & Giulietti, A.M. Duodichogamy and sex lability in Sapindaceae: the case of Paullinia weinmanniifolia . Plant Syst Evol 302, 109–120 (2016). https://doi.org/10.1007/s00606-015-1247-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00606-015-1247-5