Abstract
This work reports on a sensitive colorimetric immunoassay for aflatoxin B1 (AFB1) in foodstuff. The reagent L-ascorbic acid 2-phosphate (AAP) is added to the system, and alkaline phosphatase (ALP) hydrolyzes AAP under formation of ascorbic acid and phosphate. The ascorbic acid produced reduces chloroauric acid to form zero-valent gold (in the form of nanoparticles). Hence, Au(III) is no longer available to oxidize 2,2′-azinobis(3-ethylbenzthiazoline-6-sulfonate) (ABTS) to form a green product. Rather, the solution remains colorless. By using ALP-labelled monoclonal anti-AFB1 antibody, a competitive enzyme-label immunoassay was developed for AFB1 in a microplate coated with the AFB1-BSA conjugate. Under optimal conditions, the absorbance of the solution at 415 nm increases linearly with increasing AFB1 concentration in range from 10 pg·mL−1 to 100 ng·mL−1 (while the color gradually turns to green), and the detection limit is 7.8 pg·mL−1. The precision of the method (expressed as RSD) is ±9.7%. The accuracy was validated by analyzing both naturally contaminated and spiked food samples, and the results matched the results obtained by ELISA very well.

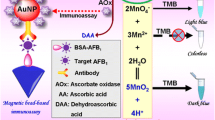
Schematic of a colorimetric aflatoxin B1 immunoassay. Antibody is labeled with alkaline phosphatase which catalyzes the hydrolysis of ascorbic acid 2-phosphate (AAP) to form ascorbic acid which reduces chloroauric acid to form gold nanoparticles. Hence, Au(III) is no longer available to oxidize 2,2′-azinobis(3-ethylbenzthiazoline-6-sulfonate) (ABTS) to form a green product.




Similar content being viewed by others
References
Lin Y, Zhou Q, Lin Y, Tang D, Niessner R, Knopp D (2015) Enzymatic hydrolysate-induced displacement reaction with multifunctional silica beads doped with horseradish peroxidase thionine conjugate for ultrasensitive electrochemical immunoassay. Anal Chem 87:8531–8540
Du B, Wang P, Xiao C, Zhou Y, Wu L, Zhao H, Su X, Yang J, He Y (2016) Antibody-free colorimetric determination of total aflatoxins by mercury(II)-mediated aggregation of lysine-functionalized gold nanoparticles. Microchim Acta 183:1493–1500
Kim M, Ye Y, Woo M, Lee J, Park H (2014) A highly efficient colorimetric immunoassay using a nanocomposite entrapping magnetic and platinum nanoparticles in ordered mesoporous carbon. Adv Healthcare Mater 3:36–41
Gao Z, Hou L, Xu M, Tang D (2014) Enhanced colorimetric immunoassay accompanying with enzyme cascade amplification strategy for ultrasensitive detection of low-abundance protein. Sci Rep 4:3966–3966
Kangas M, Burks R, Atwater J, Lukowicz R, Williams P, Holmes A (2017) Colorimetric sensor arrays for the detection and identification of chemical weapons. Crit Rev Anal Chem 47:138–153
Zarlaida F, Adlim M (2017) Gold and silver nanoparticles and indicator dyes as active agents in colorimetric spot and strip tests for mercury(II) ions: a review. Microchim Acta 184:45–58
Chen X, Wang F, Hyun J, Wei T, Qiang J, Ren X, Shin I, Yoon J (2016) Recent progress in the development of fluorescent, luminescent and colorimetric probes for detection of reactive oxygen and nitrogen species. Chem Soc Rev 45:2976–3016
Shi H, Yang L, Zhou X, Bai J, Gao J, Jia H, Li Q (2017) A gold nanoparticle-based colorimetric strategy coupled to duplex-specific nuclease signal amplification for the detection of microRNA. Microchim Acta 184:525–531
Singh S, Tripathi P, Kumar N, Nara S (2016) Colorimetric sensing of malathion using palladium-gold bimetallic nanoenzyme. Biosens Bioelectron 92:180–286
Sun J, Wang B, Zhao X, Li Z, Yang X (2016) Fluorescent and colorimetric dual-readout assay for inorganic pyrophosphatase with Cu2+-triggered oxidation of o-phyenylenediamine. Anal Chem 88:1355–1361
Chen J, Chen Q, Chen J, Qiu H (2016) Magnetic carbon nitride nanocomposites as enhanced peroxidase mimetics for use in colorimetric bioassays, and their application to the determination of H2O2 and glucose. Microchim Acta 183:3191–3199
Zhang B, Tang D, Goryacheva I, Niessner R, Knopp D (2013) Anodic-stripping voltammetric immunoassay for ultrasensitive detection of low-abundance proteins using quantum dot aggregated hollow micospheres. Chem Eur J 19:2496–2503
Huang W, Deng Y, He Y (2017) Visual colorimetric sensor array for discrimination of antioxidants in serum using MnO2 nanosheets triggered multicolour chromogenic system. Biosens Bioelectron 91:89–94
Zhang Z, Chen Z, Cheng F, Zhang Y, Chen L (2017) Highly sensitive on-site detection of glucose in human urine with naked eye based on enzymatic-like reaction mediated etching of gold nanorods. Biosens Bioelectron 89:932–936
Chen X, Zhao Q, Zou W, Qu Q, Wang F (2017) A colorimetric Fe3+ sensor based on an anionic poly(3.4-propylenedioxythiophene) derivative. Sens Actu B 244:891–896
González-Fuenzalida R, Moliner-Martínez Y, González-Béjar M, Molons-Legua C, Verdú-Andres J, Pérez-Prieto J, Campins-Falcó P (2013) In situ colorimetric quantification of silver cations in the presence of silver nanoparticles. Anal Chem 85:10013–10016
Hu Q, Zhou B, Dang P, Li L, Kong J, Zhang X (2017) Facile colorimetric assay of alkaline phosphatase activity using Fe(II)-phenanthroline reporter. Anal Chim Acta 950:170–177
Hu Q, Zhou B, Li F, Kong J, Zhang X (2016) Turn-on colorimetric platform for dual activity detection of acid and alkaline phosphatase in human whole blood. Chem Asian J 11:3040–3045
Iha M, Mini C, Okada I, Briganti R, Trucksess M (2017) The use of regenerated immunoaffinity columns for aflatoxins B1, B2, G1 and G2 in peanut confection. J Chromatogr A 1483:1–7
Wang X, Niessner R, Tang D, Knopp D (2016) Nanoparticle-based immunoasensors and immunoassays for aflatoxins. Anal Chim Acta 912:10–23
Hermanson GT (2008) Bioconjugate Techniques, 2nd (ed). Pierce Biotechnology. Thermo Fisher Scientific, Rockford, pp 798–799
Lin Y, Zhou Q, Tang D, Niessner R, Yang H, Knopp D (2016) Silver nanolabels-assisted ion-exchange reaction with CdTe quantum dots mediated exciton trapping for signal-on photoelectrochemical immunoassay of mycotoxins. Anal Chem 88:7858–7866
Acknowledgements
Support by the National Natural Science Foundation of China (21475025), the People’s Livelihood Science and Technology Innovation Project of Chongqing City (cstc2016shmszx20001), and the Advanced Natural Science Foundation of Chongqing City (cstc2015jcyjBX0126) is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The author(s) declare that they have no competing interests.
Electronic supplementary material
ESM 1
(DOC 194 kb)
Rights and permissions
About this article
Cite this article
Tang, Y., Lai, W., Zhang, J. et al. Competitive photometric and visual ELISA for aflatoxin B1 based on the inhibition of the oxidation of ABTS. Microchim Acta 184, 2387–2394 (2017). https://doi.org/10.1007/s00604-017-2268-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00604-017-2268-2