Abstract
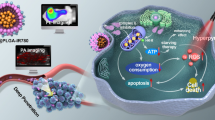
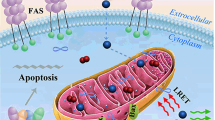
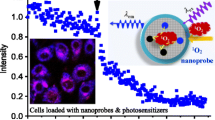
We report on a novel kind of mitochondria-targeted theranostic nanoparticles (NPs). The NPs are doped with the oxygen-sensitive probe Pt(II)-porphyrins (PtTFPP) which exerts a dual role in acting as a diagnostic tool that can sense oxygen via quenching of luminescence, but also acts as an agent in photodynamic therapy (PDT) of cancer. In addition, it allows therapeutic efficacy to be assessed in-situ. Upon appropriate high-energy photoirradiation, the NPs generate singlet oxygen by energy transfer from triplet PtTFPP to ground state oxygen, and cell death is induced via PDT. Under low-energy light irradiation, in contrast, the NPs can be utilized to detect oxygen consumption rate via time-resolved luminescence measurements in order to study the efficacy of PDT. This is the first report where a single nanoagent is used to stimulate PDT and also to assess the efficacy of PDT. In our perception, the method provides a promising platform for testing anti-cancer drugs.

We report on a kind of mitochondria-targeted theranostic nanoparticles (NPs) doped with Pt(II)-porphyrins for oxygen sensing via quenching of luminescence, efficient photodynamic therapy (PDT) of cancer and quantitative assessment of therapeutic response in-situ.






Similar content being viewed by others
References
McCarthy JR, Jaffer FA, Weissleder R (2006) A macrophage-targeted theranostic nanoparticle for biomedical applications. Small 2:983–987
Sumer B, Gao JM (2008) Theranostic nanomedicine for cancer. Nanomedicine 3:137–140
Syamchand SS, Sony G (2015) Multifunctional hydroxyapatite nanoparticles for drug delivery and multimodal molecular imaging. Microchim Acta 182:1567–1589
Lim EK, Kim T, Paik S, Haam S, Huh YM, Lee K (2015) Nanomaterials for theranostics: recent advances and future challenges. Chem Rev 115:327–394
Yang Y (2014) Upconversion nanophosphors for use in bioimaging, therapy, drug delivery and bioassays. Microchim Acta 181:263–294
Gohy JF, Zhao Y (2013) Photo-responsive block copolymer micelles: design and behavior. Chem Soc Rev 42:7117–7129
Dougherty TJ (1987) Photosensitizers: therapy and detection of malignant tumors. Photochem Photobiol 45:879–889
Henderson BW, Dougherty TJ (1992) How does photodynamic therapy work? Photochem Photobiol 55: 145–157.
Dellinger M, Ricchelli F, Moreno G, Salet C (1994) Hemato-porphyrin derivative (Photofrin) photodynamic action on Ca2+ transport in monkey kidney cells (CV-1. Photochem Photobiol 60:368–372
Henze K, Martin W (2003) Evolutionary biology: essence of mitochondria. Nature 426:127–128
Fulda S, Galluzzi L, Kroemer G (2010) Targeting mitochondria for cancer therapy. Nat Rev Drug Discov 9:447–464
Hilf R (2007) Mitochondria are targets of photodynamic therapy. J Bioenerg Biomembr 39:85–89
Morgan J, Oseroff AR (2001) Mitochondria-based photodynamic anti-cancer therapy. Adv Drug Deliv Rev 49:71–86
Li Y, Tan CP, Zhang W, He L, Ji LN, Mao ZW (2015) Phosphorescent iridium(III)-bis-N-heterocyclic carbene complexes as mitochondria-targeted theranostic and photodynamic anticancer agents. Biomaterials 39:95–104
Ye RR, Tan CP, He L, Chen MH, Jia LN, Mao ZW (2014) Cyclometalated Ir(III) complexes as targeted theranostic anticancer therapeutics: combining HDAC inhibition with photodynamic therapy. Chem Commun 50:10945–10948
Shi HF, Ma X, Zhao Q, Liu B, Qu QY, An ZF, Zhao YL, Huang W (2014) Ultrasmall Phosphorescent Polymer Dots for Ratiometric Oxygen Sensing and Photodynamic Cancer Therapy. Adv Funct Mater 24:4823–4830
Li SP, Lau CT, Louie MW, Lam YW, Cheng SH, Lo KK (2013) Mitochondria-targeting cyclometalated iridium(III)-PEG complexes with tunable photodynamic activity. Biomaterials 34:7519–7532
Cheng SH, Lee CH, Yang CS, Tseng FG, Moud CY, Lo LW (2009) Mesoporous silica nanoparticles functionalized with an oxygen-sensing probe for cell photodynamic therapy: potential cancer theranostics. J Mater Chem 19:1252–1257
Liu JP, Chen Y, Li GY, Zhang PY, Jin CZ, Zeng LL, Ji LN, Chao H (2015) Ruthenium(II) polypyridyl complexes as mitochondria-targeted two-photon photodynamic anticancer agents. Biomaterials 56:140–153
Truillet C, Lux F, Moreau J, Four M, Sancey L, Chevreux S, Boeuf G, Perriat P, Frochot C, Antoine R, Dugourd P, Portefaix C, Hoeffel C, Barberi-Heyob M, Terryn C, van GL, Lemercier G, Tillement O (2013) Bifunctional polypyridyl-Ru(II) complex grafted onto gadolinium-based nanoparticles for MR-imaging and photodynamic therapy. Dalton Trans 42:12410–12420
Varchola J, Huntosova V, Jancura D, Wagnières G, Miskovsky P, Bánó G (2014) Temperature and oxygen-concentration dependence of singlet oxygen production by RuPhen as induced by quasi-continuous excitation. Photochem Photobiol Sci 13:1781–1787
Farhadi A, Roxin Á, Wilson BC, Zheng G (2015) Nano-enabled SERS reporting photosensitizers. Theranostics 5:469–476
Neumeyer A, Bukowski M, Veith M, Lehr CM, Daum N (2014) Non-invasive determination of cellular oxygen consumption as novel cytotoxicity assay for nanomaterials. Nanotoxicology 8:50–60
Dmitriev RI, Papkovsky DB (2015) Intracellular probes for imaging oxygen concentration: how good are they? Methods Appl Fluoresc 3:034001
Papkovsky DB, Dmitriev RI (2013) Biological detection by optical oxygen sensing. Chem Soc Rev 42:8700–8732
Wang XD, Wolfbeis OS (2014) Optical methods for sensing and imaging oxygen: materials, spectroscopies and applications. Chem Soc Rev 43:3666–3761
Peng HS, Chiu DT (2015) Soft fluorescent nanomaterials for biological and biomedical imaging. Chem Soc Rev 44:4699–4722
Jin I, Yuji M, Yoshinori N, Makoto K, Mikio M (2008) Anti-tumor effect of PDT using Photofrin in a mouse angiosarcoma model. Arch Dermatol Res 300:161–166
Biswas S, Dodwadkar NS, Piroyan A, Torchilin VP (2012) Surface conjugation of triphenylphosphonium to target poly(amidoamine) dendrimers to mitochondria. Biomaterials 33:4773–4782
Wang XH, Peng HS, Yang L, You FT, Teng F, Tang AW, Zhang FJ, Li XH (2013) Poly-L-lysine assisted synthesis of core-shell nanoparticles and conjugation with triphenylphosphonium to target mitochondria. J Mater Chem B 1:5143–5152
Wang XH, Peng HS, Yang L, You FT, Teng F, Hou LL, Wolfbeis OS (2014) Targetable phosphorescent oxygen nanosensors for the assessment of tumor mitochondrial dysfunction by monitoring the respiratory activity. Angew Chem Int Ed 126:12679–12683
Wang XH, Peng HS, Ding H, You FT, Huang SH, Teng F, Dong B, Song HW (2012) Biocompatible fluorescent core-shell nanoparticles for ratiometric oxygen sensing. J Mater Chem 22:16066–16071
Wang XH, Peng HS, Chang Z, Hou LL, You FT, Teng F, Song HW, Dong B (2012) Synthesis of ratiometric fluorescent nanoparticles for sensing oxygen. Microchim Acta 178:147–152
Hu W, Kavanagh JJ (2003) Anticancer therapy targeting the apoptotic pathway. Lancet Oncol 4:721–729
O’Riordan TC, Zhdanov AV, Ponomarev GV, Papkovsky DB (2007) Analysis of intracellular oxygen and metabolic responses of mammalian cells by time-resolved Fluorometry. Anal Chem 79:9414–9419
Acknowledgments
This work was financially supported by the National Basic Research Program of China (973 Program) under grant No.2014CC339900 and Chinese Postdoctoral Science Foundation under grant No.2016 M591126.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
ESM 1
(DOCX 728 kb)
Rights and permissions
About this article
Cite this article
Wang, XH., Peng, HS., Yang, W. et al. Mitochondria-targeted theranostic nanoparticles for optical sensing of oxygen, photodynamic cancer therapy, and assessment of therapeutic efficacy. Microchim Acta 183, 2723–2731 (2016). https://doi.org/10.1007/s00604-016-1917-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00604-016-1917-1