Abstract
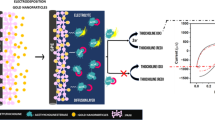
A glassy carbon electrode (GCE) was modified with a nanocomposite consisting of tetraoctylammonium bromide (TOAB), C60 fullerene, and palladium nanorods (PdNRs). The PdNRs were hydrothermally prepared and had a typical width of 20 ± 2 nm. The nanocomposite forms stable films on the GCE and exhibits a reversible redox pair for the C60/C60 − system while rendering the surface to be positively charged. The modified GCE was applied to fabricate an electrochemical biosensor for detecting acetylcholinesterase (AChE) by measurement of the amount of thiocholine formed from acetylthiocholine, best at a working voltage of −0.19 V (vs. SCE). The detection scheme is based on (a) measurement of the activity of ethyl paraoxon-inhibited AChE, and (b) measurement of AChE activity after reactivation with pralidoxime (2-PAM). Compared to the conventional methods using acetylthiocholine as a substrate, the dual method presented here provides data on the AChE activity after inhibition and subsequent reactivation, thereby yielding credible data on reactivated enzyme activity. The linear analytical range for AChE activity extends from 2.5 U L−1 to 250 kU·L−1, and the detection limit is 0.83 U L−1.

Cyclovoltammetric acetylcholinesterase (AChE) activity assay is constructed based on the palladium nanorods composited with functionalized C60 fullerene (PdNR/C60 + TOAB), which aims to measure the signal change between ethyl paraoxon-inhibited and subsequent pralidoxime (2-PAM)-reactivated AChE activity.





Similar content being viewed by others
References
Liao DL, Chen J, Zhou HP, Wang Y, Li YX, Yu C (2013) In situ formation of metal coordination polymer: a strategy for fluorescence turn-on assay of acetylcholinesterase activity and inhibitor screening. Anal Chem 85:2667–2672
Celebanska A, Lesniewski A, Niedziolka-Jonsson J, Kominiak M, Nogala W, Wittstock G, Opallo M (2014) Carbon nanoparticulate film electrode prepared by electrophoretic deposition. Electrochemical oxidation of thiocholine and topography imaging with SECM equipment in dry conditions. Electrochim Acta 144:136–140
Miao YQ, He NY, Zhu JJ (2010) History and new developments of assays for cholinesterase activity and inhibition. Chem Rev 110:5216–5234
Wang Y, Hu QZ, Guo YX, Yu L (2015) A cationic surfactant-decorated liquid crystal sensing platform for simple and sensitive detection of acetylcholinesterase and its inhibitor. Biosens Bioelectron 72:25–30
Fenzl C, Genslein C, Zopfl A, Baeumner AJ, Hirsch T (2015) A photonic crystal based sensing scheme for acetylcholine and acetylcholinesterase inhibitors. J Mater Chem B 3:2089–2095
Esposito EX, Stouch TR, Wymore T, Madura JD (2014) Exploring the physicochemical properties of oxime-reactivation rherapeutics for cyclosarin, sarin, tabun, and VX inactivated acetylcholinesterase. Chem Res Toxicol 27:99–110
Kalisiak J, Ralph EC, Zhang J, Cashman JR (2011) Amidine-oximes: reactivators for organophosphate exposure. J Med Chem 54:3319
Ye C, Wang MQ, Zhong X, Chen SH, Chai YQ, Yuan R (2016) Highly sensitive electrochemiluminescenc assay of acetylcholinesterase activity based on dual biomarkers using Pd–Au nanowires as immobilization platform. Biosens Bioelectron 79:34–40
Williams P, Sorribas A, Howes MJR (2011) Natural products as a source of Alzheimer’s drug leads. Nat Prod Rep 28:48–77
Lu DL, Shao GC, Du D, Wang J, Wang LM, Wang WJ, Lin YH (2011) Enzyme entrapped nanoporous scaffolds formed through flow-induced gelation in a microfluidic filter device for sensitive biosensing of organophosphorus compounds. Lab Chip 11:381–384
Wang J, Yokokawa M, Satake T, Suzuki H (2015) A micro IrOx potentiometric sensor for direct determination of organophosphate pesticides. Sensors Actuators B Chem 220:859–863
Gao X, Tang GC, Su XG (2012) Optical detection of organophosphorus compounds based on Mn-doped ZnSe d-dot enzymatic catalytic sensor. Biosens Bioelectron 36:75–80
Liang H, Song DD, Gong JM (2014) Signal-on electrochemiluminescence of biofunctional CdTe quantum dots for biosensing of organophosphate pesticides. Biosens Bioelectron 53:363–369
Chen YH, Hung HH, Huang MH (2009) Seed-mediated synthesis of palladium nanorods and branched nanocrystals and their use as recyclable Suzuki coupling reaction catalysts. J Am Chem Soc 131:9114–9121
Zhang H, Jin MS, Xiong YJ, Lim B, Xia YN (2013) Shape-controlled synthesis of Pd nanocrystals and their catalytic applications. Acc Chem Res 46:1783–1794
Liu W, Herrmann AK, Bigall CN, Rodriguez P, Wen D, Oezaslan M, Schmidt JT, Gaponik N, Eychmüller A (2015) Noble metal aerogels synthesis, characterization, and application as electrocatalysts. Acc Chem Res 48:154–162
Lim B, Jiang MJ, Tao J, Camargo PHC, Zhu YM, Xia YN (2009) Shape-controlled synthesis of Pd nanocrystals in aqueous solutions. Adv Funct Mater 19:189–200
Ye C, Zhong X, Yuan R, Chai YQ (2014) A novel ECL biosensor based on C60 embedded in tetraoctylammonium bromide for the determination of glucose. Sensors Actuators B Chem 199:101–107
Kroto HW, Heath JR, O,Brien SC, Curl RF, Smalley RE, (1985) C60: buckminsterfullerene. Nature 318: 162–163
Lopez AL, Mateo-Alonso A, Prato M (2011) Materials chemistry of fullerene C60 derivatives. J Mater Chem 21:1305–1318
Ye C, Zhong X, Chai YQ, Yuan R (2015) Sensing glucose based on its affinity for concanavalin a on a glassy carbon electrode modified with a C60 fullerene nanocomposite. Microchim Acta 182:2215–2221
Arayachukeat S, Palaga T, Wanichwecharungruang SP (2012) Clusters of carbon nanospheres derived from graphene oxide. ACS Appl Mater Interfaces 4:6808–6815
Fortner JD, Kim DI, Boyd AM, Falkner JC, Moran S, Colvin VL, Hughes JB, Kim JH (2007) Reaction of water-stable C60 aggregates with ozone, environ. Sci. Technol. Environ Sci Technol 41:7497–7502
Lee CY, Harbers GM, Grainger DW, Gamble LJ, Castner DG (2007) Fluorescence, XPS, and TOF-SIMS surface chemical state image analysis of DNA microarrays. J Am Chem Soc 129:9429–9438
Papirer E, Lacroix R, Donnet JB, Nanse G, Fioux P (1994) XPS study of the halogenation of carbon black-part 1. Carbo 32:1341–1358
Nakashima N, Tokunaga T, Nonaka Y, Nakanishi T, Murakami H, Sagara T (1998) A fullerene/lipid electrode device: reversible electron transfer reaction of C60 embedded in a cast film of an artificial ammonium lipid on an electrode in aqueous solution. Angew Chem Int Ed 37:2671–2673
Nakashima N, Ishii T, Shirakusa M, Nakanishi T, Murakami H, Sagara T (2001) Molecular bilayer-based superstructures of a fullerene-carrying ammonium amphiphile: structure and electrochemistry. Chem Eur J 7:1766–1772
Acknowledgments
This work was financially supported by the NNSF of China (21575116, 21275119, and 51473136), Natural Science Foundation of Chongqing City (CSTC-2014JCYJA20005), and the Fundamental Research Funds for the Central Universities (XDJK2015A002), China.
Author information
Authors and Affiliations
Corresponding authors
Electronic Supplementary Material
ESM 1
(DOC 1446 kb)
Rights and permissions
About this article
Cite this article
Ye, C., Zhong, X., Wang, MQ. et al. Cyclovoltammetric acetylcholinesterase activity assay after inhibition and subsequent reactivation by using a glassy carbon electrode modified with palladium nanorods composited with functionalized C60 fullerene. Microchim Acta 183, 2403–2409 (2016). https://doi.org/10.1007/s00604-016-1888-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00604-016-1888-2