Abstract
We describe an electrochemical aptasensor for the amino acid homocysteine (hCys). A gold electrode was modified with a highly specific aptamer against hCys (a 66-base DNA oligonucleotide) acting as the recognition probe. The method is highly selective over cysteine and methionine. The effects of accumulation time, type and concentration of accumulation buffer and pH, type and concentration of stripping buffer were studied. Under optimized conditions and a working potential of 1.07 V (vs. Ag/AgCl), the response to hCys is linear in the 0.2 to 10 μM concentration range. The detection limit is 10 nM, and the relative standard deviation is 3.1 % (at 1 μM of hCys). The electrode can be regenerated by immersing it into a 3 M solution of urea solution. The method was applied to the determination of hCys in (spiked) serum and urine and gave recoveries of 88.5 and 96.5 %, respectively.

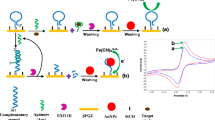
A label-free aptasensor for electrochemical detection of homocysteine was designed. A gold electrode was modified with a thiolated aptamer by self-assembly. The assembled interface enables detection of homocysteine by differential pulse voltammetry.





Similar content being viewed by others
References
Finkelstein JD, Martin JJ (2000) Homocysteine. Int J Biochem Cell Biol 32:385–389
Savage DG, Lindenbaum J, Stabler SP, Allen RH (1994) Sensitivity of serum methylmalonic acid and total homocysteine determinations for diagnosing cobalamin and folate deficiencies. Am J Med 96(3):239–246
Stabler S, Marcell P, Podell E, Allen R, Savage D, Lindenbaum J (1998) Elevation of total homocysteine in the serum of patients with cobalamin or folate deficiency detected by capillary gas chromatography-mass spectrometry. J Clin Invest 81(2):466–474
Rasmussen K, Mùller J (2000) Total homocysteine measurement in clinical practice. Ann Clin Biochem 37:627–648
Finkelstein JD (1990) Methionine metabolism in mammals. J Nutr Biochem 1:228–237
Zhang G, Liu D, Shuang S, Choi MMF (2006) A homocysteine biosensor with eggshell membrane as an enzyme immobilization platform. Sensors Actuators B 114(2):936–942
Refsum H, Ueland PM, Nygard O, Vollset SE (1998) Homocysteine and cardiovascular disease. Annu Rev Med 49:31–62
Nekrassova O, Lawrence NS, Compton RG (2003) Analytical determination of homocysteine: a review. Talanta 60:1085–1095
Frick B, Schrocksnadel K, Neurauter G, Wirleitner B, Artner-Dworzaka E, Fuchs D (2003) Rapid measurement of total plasma homocysteine by HPLC. Clin Chim Acta 331:19–23
Głowacki R, Borowczyk K, Bald E, Jakubowski H (2010) On-column derivatization with o-phthaldialdehyde for fast determination of homocysteine in human urine. Anal Bioanal Chem 396(6):2363–2366
Yao X, Wang Y, Chen G (2007) Simultaneous determination of aminothiols, ascorbic acid and uric acid in biological samples by capillary electrophoresis with electrochemical detection. Biomed Chromatogr 21:520–526
Windelberg A, Arseth O, Kvalheim G, Ueland PM (2005) Automated assay for the determination of methylmalonic acid, total homocysteine, and related amino acids in human serum or plasma by means of methylchloroformate derivatization and gas chromatography–mass spectrometry. Clin Chem 51(11):2103–2109
Andersson A, Brattström L, Isaksson A, Israelsson B, Hultberg B (1989) Determination of homocysteine in plasma by ion-exchange chromatography. Scand J Clin Lab Invest 49:445–449
Leesutthiphonchai W, Dungchai W, Siangproh W, Ngamrojnavanich N, Chailapakul O (2011) Selective determination of homocysteine levels in human plasma using a silver nanoparticle-based colorimetric assay. Talanta 85(2):870–876
Gui R, Wang Y, Sun J (2014) Protein-stabilized fluorescent nanocrystals consisting of a gold core and a silver shell for detecting the total amount of cysteine and homocysteine. Microchim Acta 181(11):1231–1238
Guo Y, Yang L, Li W, Wang X, Shang Y, Li B (2016) Carbon dots doped with nitrogen and sulfur and loaded with copper(II) as a “turn-on” fluorescent probe for cystein, glutathione and homocysteine. Microchim. Acta, First online: 12 February 2016
Rusin O, Luce NNS, Agbaria RA, Escobedo JO, Jiang S, Warner IM, Dawan FB, Lian K, Strongin RM (2004) Visual detection of cysteine and homocysteine. J Am Chem Soc 126(2):438–439
Frantzen F, Faaren AL, Alfheim I, Nordhei AK (1998) Enzyme conversion immunoassay for determining total homocysteine in plasma or serum. Clin Chem 44(2):311–316
Lee PT, Lowinsohn D, Compton RG (2014) The selective electrochemical detection of homocysteine in the presence of glutathione, cysteine, and ascorbic acid using carbon electrodes. Analyst 139:3755–3762
Tuerk C, Gold L (1990) Systematic evolution of ligands by exponential enrichment: RNA ligands to bacteriophage T4 DNA polymerase. Science 249:505–510
Ellington AD, Szostak JW (1990) In vitro selection of RNA molecules that bind specific ligands. Nature 346:818–822
Zhang YL, Huang Y, Jiang JH, Shen GL, Yu RQ (2007) Electrochemical aptasensor based on proximity-dependent surface hybridization assay for single-step, reusable, sensitive protein detection. J Am Chem Soc 129:15448–15449
Chen D, Yao D, Xie C, Liu D (2014) Development of an aptasensor for electrochemical detection of tetracycline. Food Control 42:109–115
Song W, Li H, Liang H, Qiang W, Xu D (2014) Disposable electrochemical aptasensor array by using in situ DNA hybridization inducing silver nanoparticles aggregate for signal amplification. Anal Chem 86(5):2775–2783
Huang L, Wu J, Zheng L, Qian H, Xue F, Wu Y, Pan D, Adeloju SB, Chen W (2013) Rolling chain amplification based signal-enhanced electrochemical aptasensor for ultrasensitive detection of ochratoxin A. Anal Chem 85(22):10842–10849
Xue F, Wu J, Chu H, Mei Z, Ye Y, Liu J, Zhang R, Peng C, Zheng L, Chen W (2013) Electrochemical aptasensor for the determination of bisphenol A in drinking water. Microchim Acta 180:109–115
McKeague M, Foster A, Miguel Y, Giamberardino A, Verdin C, Chan JYS, DeRosa MC (2013) Development of a DNA aptamer for direct and selective homocysteine detection in human serum. RSC Adv 3:24415–24422
Luo L, Zhang Z, Ding Y, Deng D, Zhub X, Wang Z (2013) Label-free electrochemical impedance genosensor based on 1-aminopyrene/graphene hybrids. Nanoscale 5:5833–5840
Yang X, Bing T, Mei H, Fang C, Caoa Z, Shangguan D (2011) Characterization and application of a DNA aptamer binding to L-tryptophan. Analyst 136:577–585
Mohamadi M, Mostafavi A, Mahani MT (2015) Electrochemical determination of biophenol oleuropein using a simple label-free DNA biosensor. Bioelectrochemistry 101:52–57
Salehzadeh H, Mokhtari B, Nematollahi D (2014) Selective electrochemical determination of homocysteine in the presence of cysteine and glutathione. Electrochim Acta 123:353–361
Fouladgar M, Mohammadzadeh S, Nayeri H (2014) Electrochemical determination of homocysteine using carbon nanotubes modified paste electrode and isoprenaline as a mediator. Russ J Electrochem 50(10):981–988
Acknowledgments
This work was supported by grants from the Research Council of Shahid Bahonar University of Kerman.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no competing interests
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOC 743 kb)
Rights and permissions
About this article
Cite this article
Saeed, J., Mirzaei, M. & Torkzadeh-Mahani, M. A selective and regenerable voltammetric aptasensor for determination of homocysteine. Microchim Acta 183, 2205–2210 (2016). https://doi.org/10.1007/s00604-016-1852-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00604-016-1852-1