Abstract
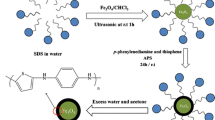
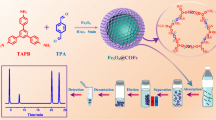
Core-shell nanoparticles (NPs) of the type Fe3O4@SiO2 were prepared, coated with 3 different ionic liquids, and tested for their capability of extracting bisphenol A (BPA). The results showed BPA is best extracted by NPs of the type Fe3O4@SiO2@[OMIM] (where OMIM stands for 1-octyl-3-methylimidazole hexafluorophosphate). Following desorption with methanol, BPA was quantified by HPLC with fluorescence detection in the UV. The following figures of merit were found under optimal conditions: A linear range from 0.5 to 2.0 × 104 μg·L‾1, a detection limit of 90 ng·L‾1, a relative standard deviation of 1.2 % (for n = 3 and 10 μg·L‾1), and a pre-concentration factor of 25. The NPs can be re-used up to 10 times. The method was successfully applied to the determination of BPA in plastic tableware.

0.10 g Fe3O4@SiO2@ILs were added to 40 mL sample solution (pH = 10.0), and the mixture was equilibrated for 10 min at 35 °C. The bisphenol A (BPA)-adsorbed Fe3O4@SiO2@ILs were separated by an external magnetic field, eluted with 4.0 mL of methanol, then 20 μL eluate was injected into HPLC system for the detection BPA.




Similar content being viewed by others
References
Li G, Lu YT, Lu C, Zhu MS, Zhai CY, Du YK, Yang P (2015) Efficient catalytic ozonation of bisphenol-A over reduced grapheme oxide modified sea urchin-like α-MnO2 architectures. J Hazard Mater 294:201
Abhishek P, Raju PS, Kaustubha M (2013) Selective separation of bisphenol A from aqueous solution using supported ionic liquid membrane. Sep Purif Technol 107:70
Parka HS, Koduru JR, Choo KH, Lee B (2015) Activated carbons impregnated with iron oxide nanoparticles for enhanced removal of bisphenol A and natural organic matter. J Hazard Mater 286:315
Najafi M, Khalilzadeh MA, Karimi-Maleh H (2014) A new strategy for determination of bisphenol A in the presence of Sudan I using a ZnO/CNTs/ionic liquid paste electrode in food samples. Food Chem 158:125
Zhou QX, Gao YY, Xie GH (2011) Determination of bisphenol A, 4-n-nonylphenol, and 4-tert-octylphenol by temperature-controlled ionic liquid dispersive liquid-phase microextraction combined with high performance liquid chromatography-fluorescence detector. Talanta 85:1598
Santhi VA, Hairin T, Mustafa AM (2012) Simultaneous determination of organochlorine pesticides and bisphenol A in edible marine biota by GC–MS. Chemosphere 86:1066
Mei SR, Wu D, Jiang M, Lu B, Lim JM, Zhou YK, Lee YI (2011) Determination of trace bisphenol A in complex samples using selective molecularly imprinted solid-phase extraction coupled with capillary electrophoresis. Microchem J 98:150
Xu JY, Li Y, Bie JX, Jiang W, Guo JJ, Luo YL, Shen F, Sun CY (2015) Colorimetric method for determination of bisphenol A based on aptamer-mediated aggregation of positively charged gold nanoparticles. Microchim Acta 182:2131
Sadeghi S, Azhdari H, Arabib H, Moghaddam AZ (2012) Surface modified magnetic Fe3O4 nanoparticles as a selective sorbent for solid phase extraction of uranyl ions from water samples. J Hazard Mater 215–216:208
Ding MJ, Wu XL, Yuan LH, Wang S, Li Y, Wang RY, Wen TT, Du SH, Zhou XM (2011) Synthesis of core–shell magnetic molecularly imprinted polymers and detection of sildenafil and vardenafil in herbal dietary supplements. J Hazard Mater 191:177
Geng YY, Ding MY, Chen H, Li HF, Lin JM (2012) Preparation of hydrophilic carbon-functionalized magnetic microspheres coated with chitosan and application in solid-phase extraction of bisphenol A in aqueous samples. Talanta 89:189
Li SZ, Gong YB, Yang YC, He C, Hu LL, Zhu LF, Sun LP, Shu D (2015) Recyclable CNTs/Fe3O4 magnetic nanocomposites as adsorbents to remove bisphenol A from water and their regeneration. Chem Eng J 260:231
Gong AQ, Ping WH, Wang J, Zhu XS (2014) Cyclodextrin polymer/Fe3O4 nanocomposites as solid phase extraction material coupled with UV–vis spectrometry for the analysis of ruti. Spectrochim Acta A 122:331
Yang J, Si L, Cui SH, Bi WT (2015) Synthesis of a graphitic carbon nitride nanocomposite with magnetite as a sorbent for solid phase extraction of phenolic acids. Microchim Acta 182(3):737
Deng N, Li M, Zhao LJ, Lu CF, Rooy SL, Warner IM (2011) Highly efficient extraction of phenolic compounds by use of magnetic room temperature ionic liquids for environmental remediation. J Hazard Mater 192:1350
Berton P, Regmi BP, Spivak DA, Warner IM (2014) Ionic liquid-based dispersive microextraction of nitrotoluenes in water samples. Microchim Acta 181(11):1191
Wang R, Yuan Y, Yang X, Han Y, Yan H (2015) Polymethacrylate microparticles covalently functionalized with an ionic liquid for solid-phase extraction of fluoroquinolone antibiotics. Microchim Acta 182(13):2201
Ramandi NF, Shemirani F, Farahani MD (2014) Dispersive solid phase extraction of lead (II) using a silica nanoparticle-based ionic liquid ferrofluid. Microchim Acta 181(15):1833
Abolghasemi MM, Yousefi V, Piryaei M (2015) Double-charged ionic liquid-functionalized layered double hydroxide nanomaterial as a new fiber coating for solid-phase microextraction of phenols. Microchim Acta 182(13):2155
Zheng XY, He LJ, Duan YJ, Jiang XM, Xiang GQ, Zhao WJ, Zhang SS (2014) Poly(ionic liquid) immobilized magnetic nanoparticles as new adsorbent for extraction and enrichment of organophosphorus pesticides from tea drinks. J Chromatogr A 1358:39
He H, Yuan DH, Gao ZQ, Xiao DL, He H, Dai H, Peng J, Li N (2014) Mixed hemimicelles solid-phase extraction based on ionic liquid-coated Fe3O4/SiO2 nanoparticles for the determination of flavonoids in bio-matrix samples coupled with high performance liquid chromatography. J Chromatogr A 1324:78
Chen JP, Zhu XS (2015) Ionic liquid coated magnetic core/shell Fe3O4@SiO2 nanoparticles for the separation/analysis of linuron in food samples. Spectrochim Acta A 137:456
Ballesteros-Gomez A, Brandsma SH, de Boer J, Leonards PEG (2014) Analysis of two alternative organophosphorus flame retardants in electronic and plastic consumer products: resorcinol bis-(diphenylphosphate) (PBDPP) and bisphenol A bis (diphenylphosphate) (BPA-BDPP). Chemosphere 116:10
Zhu XS, Gong AQ, Yu SH (2008) Fluorescence probe enhanced spectrofluorimetric method for the determination of gatifloxacin in pharmaceutical formulations and biological fluids. Spectrochim Acta A 69:478
Russo V, Tesser R, Trifuoggi M, Giugni M, Di Serio M (2015) A dynamic intraparticle model for fluid–solid adsorption kinetics. Comput Chem Eng 74:66
Gonzalez-Centeno MR, Comas-Serra F, Femenia A, Rossello C, Simal S (2015) Effect of power ultrasound application on aqueous extraction of phenolic compounds and antioxidant capacity from grape pomace (vitis vinifera L.): experimental kinetics and modeling. Ultrason Sonochem 22:506
Liu GF, Ma J, Li XC, Qin QD (2009) Adsorption of bisphenol A from aqueous solution onto activated carbons with different modification treatments. J Hazard Mater 164:1275
Wang HL, Duan AL, Dahlgren RA, Li YY, Li CL, Wang WW, Zeng AB, Wang XD (2014) The joint effects of room temperature ionic liquids and ordered media on fluorescence characteristics of estrogens in water and methanol. Spectrochim Acta A 128:497
Zhang XF, Zhang YK, Liu LM (2014) Fluorescence lifetimes and quantum yields of ten rhodamine derivatives: structural effect on emission mechanism in different solvents. J Lumin 145:448
Lawton JS, Budil DE (2010) Electron spin resonance investigation of the effects of methanol on microscopic viscosity, ordering, and polarity in different phases of ionomer membranes with sulfonated polyarylene backbones. J Membr Sci 357:47
Acknowledgments
The authors acknowledge the financial support from the National Natural Science Foundation of China (21375117) and a project funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The author(s) declare that they have no competing interests
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOC 108 kb)
Rights and permissions
About this article
Cite this article
Chen, S., Chen, J. & Zhu, X. Solid phase extraction of bisphenol A using magnetic core-shell (Fe3O4@SiO2) nanoparticles coated with an ionic liquid, and its quantitation by HPLC. Microchim Acta 183, 1315–1321 (2016). https://doi.org/10.1007/s00604-016-1757-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00604-016-1757-z