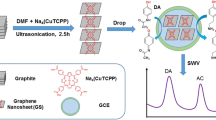
Abstract
The authors describe a glassy carbon disk electrode which after modification with poly(Alizarin Violet 3B), multiwalled carbon nanotubes and graphene enables simultaneous determination of the drugs acetaminophen (AP), theophylline (TP) and caffeine (CF). The electrochemical response to AP, TP and CF at the modified electrode was studied by cyclic voltammetry, and the results revealed an excellent electrocatalytic activity towards the oxidation of the three analytes at potentials of typically 0.5, 1.15 and 1.4 V (vs. SCE) respectively. The anodic peaks are well defined and occur at lower oxidation potential and enhanced oxidation peak currents (compared to an unmodified electrode). Simultaneous differential pulse voltammetric measurements resulted in calibration plot for AP, TP and CF were obtained that cover range from 0.2 to 100 μM for AP, from 0.5 to 120 μM for TP, and from 1.0 to 120 μM for CF. The respective detection limits are 0.01, 0.02 and 0.10 μM. The method was applied to simultaneous determination of AP, TP and CF in spiked human serum and gave satisfactory results.

A nanocomposite consisting of poly(Alizarin violet), multiwalled carbon nanotubes and graphene was used to modify a glassy carbon electrode which then can be used simultaneously determine acetaminophen, theophylline and caffeine.






Similar content being viewed by others
References
Mazer, Perrone J (2008) Acetaminophen-induced nephrotoxicity: pathophysiology, clinical manifestations, and management. J Appl Toxicol 42
Singh D, Sahu A (2006) Spectrophotometric determination of caffeine and theophylline in pure alkaloids and its application in pharmaceutical formulations. Anal Biochem 349:176
Mullen M, Whitehouse JM, Shine G, Towell A (2011) The immediate and short-term chemosensory impacts of coffee and caffeine on cardiovascular activity. Food Funct 2:197
Zhang Y, Mehrotra N, Budha NR, Christensen ML, Meibohm B (2008) A tandem mass spectrometry assay for the simultaneous determination of acetaminophen, caffeine, phenytoin, ranitidine, and theophylline in small volume pediatric plasma specimen. Clin Chim Acta 398:105
Lee CA, Lillibridge JH, Nelson SD, Slattery JT (1996) Effects of caffeine and theophylline on acetaminophen pharmacokinetics: P450 inhibition and activation. J Pharmacol Exp Ther 277:287
Goyal RN, Gupta VK, Oyama M, Bachheti N (2005) Differential pulse voltammetric determination of paracetamol at nanogold modified indium tin oxide electrode. Electrochem Commun 7:803
Bates F, del Valle M (2015) Voltammetric sensor for theophylline using sol–gel immobilized molecularly imprinted polymer particles. Microchim Acta 182:933
Meareg A, Shimelis A (2012) Polymer modified glassy carbon electrode for the electrochemical determination of caffeine in coffee. Talanta 93:122
Yang GM, Zhao FQ, Zeng BZ (2014) Facile fabrication of a novel anisotropic gold nanoparticle-chitosan-ionic liquid/graphene modified electrode for the determination of theophylline and caffeine. Talanta 127:116
Gao YY, Wang H, Guo LP (2013) Simultaneous determination of theophylline and caffeine by large mesoporous carbon/Nafion modified electrode. J Electroanal Chem 706:7
Wang ZH, Li ZG, Zhou SP (2004) Voltammetric behavior of caffeine and theophylline at poly (4-aminopyridine) modified electrode and their simultaneous determination. Chin J Anal Chem 32:305
Lu XC, Huang KJ, Wu ZW (2012) Simultaneous determination of acetaminophen and caffeine based on graphene oxide/cerium hexacyanoferrate modified glassy carbon electrode. Chin J Anal Chem 40:452
Saciloto TR, Cervini P, Cavalheiro ETG (2013) Simultaneous voltammetric determination of acetaminophen and caffeine at a graphite and polyurethane screen-printed composite electrode. J Braz Chem Soc 24:1461
Fernandes DM, Silva N, Pereira C, Moura C, Magalhaes JMCS, Bachiller-Baeza B, Freire C (2015) MnFe2O4@CNT-N as novel electrochemical nanosensor for determination of caffeine, acetaminophen and ascorbic acid. Sensors Actuators B 218:128
Liu YX, Dong XC, Chen P (2012) Biological and chemical sensors based on graphene materials. Chem Soc Rev 41:2283
Novoselov KS, Geim AK, Morozov SV, Jiang D, Zhang Y, Dubonos SV, Grigorieva IV, Firsov AA (2004) Electric field effect in atomically thin carbon films. Science 306:666
Shan CS, Tang H, Wong TL, He LF, Lee ST (2012) Facile synthesis of a large quantity of graphene by chemical vapor deposition: an advanced catalyst carrier. Adv Mater 24:2491
Guo HL, Wang XF, Qian QY, Wang FB, Xia XH (2009) A green approach to the synthesis of graphene nanosheets. ACS Nano 3:2653
Park S, Ruoff RS (2009) Chemical methods for the production of graphenes. Nat Nanotechnol 4:217
Pham VH, Pham HD, Dang TT, Hur SH, Kim EJ, Kong BS, Kim S, Chung JS (2012) Chemical reduction of an aqueous suspension of graphene oxide by nascent hydrogen. J Mater Chem 22:10530
Haque AMJ, Park H, Sung D, Jon S, Choi SY, Kim K (2012) An electrochemically reduced graphene oxide-based electrochemical immunosensing platform for ultrasensitive antigen detection. Anal Chem 84:1871
Hu FX, Chen SH, Wang CY, Yuan R, Yuan DH, Wang C (2012) Study on the application of reduced graphene oxide and multiwall carbon nanotubes hybrid materials for simultaneous determination of catechol, hydroquinone, p-cresol and nitrite. Anal Chim Acta 724:40
Li JH, Kuang DZ, Feng YL, Zhang FX, Xu ZF, Liu MQ, Wang DP (2013) Electrochemical tyrosine sensor based on a glassy carbon electrode modified with a nanohybrid made from grapheme oxide and multiwalled carbon nanotubes. Microchim Acta 180:49
Hu FX, Chen SH, Wang CY (2012) Multi-wall carbon nanotube-polyaniline biosensor based on lectin-carbohydrate affinity for ultrasensitive detection of Con A. Biosens Bioelectron 34:202
Wang Y (2011) Simultaneous determination of uric acid, xanthine and hypoxanthine at poly(pyrocatechol violet)/functionalized multi-walled carbon nanotubes composite film modified electrode. Colloids Surf, B 88:614
Wang Y, Bi CY (2013) Simultaneous electrochemical determination of ascorbic acid, dopamine and uric acid using poly (tyrosine)/functionalized multi-walled carbon nanotubes composite film modified electrode. J Mol Liq 177:26
Gao YS, Xu JK, Lu LM, Wu LP, Zhang KX, Wu Y (2014) Overoxidized polypyrrole/graphene nanocomposite with good electrochemical performance as novel electrode material for the detection of adenine and guanine. Biosens Bioelectron 62:261
Tao Y, Kong QQ, Li QH, Wang XX, Chen LH, Jiao K (2014) Highly sensitive and synergistic detection of guanine and adenine based on poly(xanthurenic acid)-reduced graphene oxide interface. ACS Appl Mater Interfaces 61:1032
Tian L, Zhang B, Sun D, Chen RZ, Wang BB, Li TJ (2014) A thin poly(acridine orange) filmcontaining reduced grapheme oxide for voltammetric simultaneous sensing of ascorbic acid and uric acid. Microchim Acta 181:589
Liang WJ, Chen YP, Zheng FY, Li SX (2014) Titanium dioxide nanoparticle based solid phase extraction of trace Alizarin Violet 3B, followed by its specrophotometric determination. Microchim Acta 181:1513
Ba X, Luo LQ, Ding YP, Zhang Z, Chu YL, Wang BJ, Ouyang XQ (2012) Poly(alizarin red)/graphene modified glassy carbon electrode for simultaneous determination of purine and pyrimidine. Anal Chim Acta 752:94
Zhou M, Wang Y, Zhai Y, Zhai J, Ren W, Wang F, Dong S (2009) Controlled synthesis of large-area and patterned electrochemically reduced graphene oxide films. Chem Eur J 15:6116
Bard AJ, Faulkner LR (1980) Electrochemical methods, fundamentals and application. Wiley, New York, p 222
Acknowledgments
The research presented in this manuscript was supported by the Natural Science Foundation of Shandong Province of China (Y2006B28).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOC 90 kb)
Rights and permissions
About this article
Cite this article
Wang, Y., Wu, T. & Bi, Cy. Simultaneous determination of acetaminophen, theophylline and caffeine using a glassy carbon disk electrode modified with a composite consisting of poly(Alizarin Violet 3B), multiwalled carbon nanotubes and graphene. Microchim Acta 183, 731–739 (2016). https://doi.org/10.1007/s00604-015-1688-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00604-015-1688-0