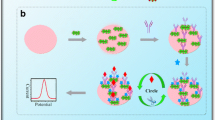
Abstract
We report on a highly sensitive electrochemical immunoassay for the serum inflammation marker amyloid A (SAA). It is making use of a glassy carbon electrode that was modified with carboxy-endcapped polypyrrole (PPy-α-COOH), multiwalled carbon nanotubes (MWCNTs), ionic liquid and chitosan acting as the support platform. The nanocomposite increases the sensitivity and stability of the assay. Antibody against SAA was immobilized on a monolayer surface consisting of PPy-α-COOH. The electrode material was characterized by scanning electron microscopy, transmission electron microscopy, Fourier transform infrared spectra, cyclic voltammetry, electrochemical impedance spectroscopy and differential pulse voltammetry. The calibration plot for this assay, when operated at 0.16 V (vs. SCE) and applied to spiked serum samples, is linear in the 0.001 to 900 ng mL−1 SAA concentration range, and the detection limit is as low as 0.3 pg mL−1 (at an S/N ratio of 3). The electrode is stable and highly sensitive. The detection scheme is likely to be applicable to numerous other kinds of immunoassays.

We describe a highly sensitive serum amyloid A (SAA) electrochemical immunosensor based on in situ polymerization of carboxy-endcapped polypyrrole (PPy-α-COOH) on multiwalled carbon nanotubes (MWCNTs), ionic liquid (IL), and chitosan (Chit) nanocomposite. Excellent performance is achieved because PPy-α-COOH offers anchor sites for covalent immobilization of anti-SAA, while the MWCNTS/IL/Chit nanocomposite warrants good electrical conductivity and high stability.





Similar content being viewed by others
References
Ridker PM, Hennekens CH, Buring JE, Rifai N (2000) C-reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women. N Engl J Med 342(12):836–843
Tamamoto T, Ohno K, Goto-Koshino Y, Fujino Y, Tsujimoto H (2012) Serum amyloid A uptake by feline peripheral macrophages. Vet Immunol Immunopathol 150(1–2):47–52
Uhlar CM, Whitehead AS (1999) Serum amyloid A, the major vertebrate acute-phase reactant. Eur J Biochem 265(2):501–523
Koutroubakis IE, Dilaveraki E, Vlachonikolis IG, Vardas E, Vrentzos G, Ganotakis E, Mouzas IA, Gravanis A, Emmanouel D, Kouroumalis EA (2000) Hyperhomocysteinemia in Greek patients with inflammatory bowel disease. Dig Dis Sci 45(12):2347–2351
Tsun JG, Shiu SW, Wong Y, Yung S, Chan TM, Tan KC (2013) Impact of serum amyloid A on cellular cholesterol efflux to serum in type 2 diabetes mellitus. Atherosclerosis 231(2):405–410
Kushner I (1988) The acute phase response: an overview. Methods Enzymol 163:373–383
Gabay C, Kushner I (1999) Acute-phase proteins and other systemic responses to inflammation. N Engl J Med 340(6):448–454
Fischer K, Theil G, Hoda R, Fornara P (2012) Serum amyloid A: a biomarker for renal cancer. Anticancer Res 32(5):1801–1804
Howard BA, Wang MZ, Campa MJ, Corro C, Fitzgerald MC, Patz EF Jr (2003) Identification and validation of a potential lung cancer serum biomarker detected by matrix-assisted laser desorption/ionization-time of flight spectra analysis. Proteomics 3(9):1720–1724
Liu DH, Wang XM, Zhang LJ, Dai SW, Liu LY, Liu JF, Wu SS, Yang SY, Fu S, Xiao XY, He DC (2007) Serum amyloid A protein: a potential biomarker correlated with clinical stage of lung cancer. Biomed Environ Sci 20(1):33–40
Rau B, Schilling MK, Beger HG (2004) Laboratory markers of severe acute pancreatitis. Dig Dis 22(3):247–257
Rau BM (2007) Predicting severity of acute pancreatitis. Curr Gastroenterol Rep 9(2):107–115
Malle E, De Beer FC (1996) Human serum amyloid A (SAA) protein: a prominent acute-phase reactant for clinical practice. Eur J Clin Invest 26(6):427–435
Timucin C, Gul O, Kutuk O, Basaga H (2012) Antibody array-based immunosensor for detecting cardiovascular disease risk markers. J Immunoassay Immunochem 33(3):275–290
Akter R, Kyun Rhee C, Rahman MA (2013) A stable and sensitive voltammetric immunosensor based on a new non-enzymatic label. Biosens Bioelectron 50:118–124
Zhai C, Sun X, Zhao W, Gong Z, Wang X (2013) Acetylcholinesterase biosensor based on chitosan/prussian blue/multiwall carbon nanotubes/hollow gold nanospheres nanocomposite film by one-step electrodeposition. Biosens Bioelectron 42:124–130
Ahuja T, Mir IA, Kumar D, Rajesh (2007) Biomolecular immobilization on conducting polymers for biosensing applications. Biomaterials 28(5):791–805
Shang F, Liu Y, Hrapovic S, Glennon JD, Luong JH (2009) Selective detection of dopamine using a combined permselective film of electropolymerized (poly-tyramine and poly-pyrrole-1-propionic acid) on a boron-doped diamond electrode. Analyst 134(3):519–527
Yuqing M, Jianrong C, Xiaohua W (2004) Using electropolymerized non-conducting polymers to develop enzyme amperometric biosensors. Trends Biotechnol 22(5):227–231
Hu W, Li CM, Cui X, Dong H, Zhou Q (2007) In situ studies of protein adsorptions on poly(pyrrole-co-pyrrole propylic acid) film by electrochemical surface plasmon resonance. Langmuir 23(5):2761–2767
Hu W, Li CM, Dong H (2008) Poly(pyrrole-co-pyrrole propylic acid) film and its application in label-free surface plasmon resonance immunosensors. Anal Chim Acta 630(1):67–74
Puri N, Mishra SK, Niazi A, Biradar AM, Rajesh (2013) Structural and impedance spectroscopic studies on biofunctionalized poly(pyrrole-co-pyrrolepropylic acid) film. Synth Met 169:18–24
Lee JW, Serna F, Schmidt CE (2006) Carboxy-endcapped conductive polypyrrole: biomimetic conducting polymer for cell scaffolds and electrodes. Langmuir 22(24):9816–9819
Katz E, Willner I (2004) Biomolecule-functionalized carbon nanotubes: applications in nanobioelectronics. ChemPhysChem 5(8):1084–1104
Shen X, Wang X, Tao S, Xing B (2014) Displacement and competitive sorption of organic pollutants on multiwalled carbon nanotubes. Environ Sci Pollut Res Int 21(20):11979–11986
Yu X, Hua T, Liu X, Yan Z, Xu P, Du P (2014) Nickel-based thin film on multiwalled carbon nanotubes as an efficient bifunctional electrocatalyst for water splitting. ACS Appl Mater Interfaces 6(17):15395–15402
Batra B, Lata S, Pundir CS (2013) Construction of an improved amperometric acrylamide biosensor based on hemoglobin immobilized onto carboxylated multi-walled carbon nanotubes/iron oxide nanoparticles/chitosan composite film. Bioprocess Biosyst Eng 36(11):1591–1599
Choudhary M, Singh A, Kaur S, Arora K (2014) Enhancing lung cancer diagnosis: electrochemical simultaneous bianalyte immunosensing using carbon nanotubes-chitosan nanocomposite. Appl Biochem Biotechnol 174(3):1188–1200
Lin J, Qu W, Zhang S (2007) Disposable biosensor based on enzyme immobilized on Au-chitosan-modified indium tin oxide electrode with flow injection amperometric analysis. Anal Biochem 360(2):288–293
Li D, Muller MB, Gilje S, Kaner RB, Wallace GG (2008) Processable aqueous dispersions of graphene nanosheets. Nat Nanotechnol 3(2):101–105
Kavosi B, Salimi A, Hallaj R, Amani K (2014) A highly sensitive prostate-specific antigen immunosensor based on gold nanoparticles/PAMAM dendrimer loaded on MWCNTS/chitosan/ionic liquid nanocomposite. Biosens Bioelectron 52:20–28
Liu H, Gao J, Xue M, Zhu N, Zhang M, Cao T (2009) Processing of graphene for electrochemical application: noncovalently functionalize graphene sheets with water-soluble electroactive methylene green. Langmuir 25(20):12006–12010
Cho S, Lee JS, Jun J, Kim SG, Jang J (2014) Fabrication of water-dispersible and highly conductive PSS-doped PANI/graphene nanocomposites using a high-molecular weight PSS dopant and their application in H2S detection. Nanoscale 6(24):15181–15195
Sun X, Shuyuan D, Xiangyou W (2012) Amperometric immusensor for carbofuran detection based on gold nanoparticles and PB-MWCNTs-CTS composite film. Eur Food Res Technol 235:469–477
Lin M, Hu X, Ma Z, Chen L (2012) Functionalized polypyrrole nanotube arrays as electrochemical biosensor for the determination of copper ions. Anal Chim Acta 746:63–69
Acknowledgments
This study was supported financially by the National Natural Science Foundation of China (81370403, 21205146).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOC 822 kb)
Rights and permissions
About this article
Cite this article
Xia, C., Li, Y., Yuan, G. et al. Immunoassay for serum amyloid A using a glassy carbon electrode modified with carboxy-polypyrrole, multiwalled carbon nanotubes, ionic liquid and chitosan. Microchim Acta 182, 1395–1402 (2015). https://doi.org/10.1007/s00604-015-1465-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00604-015-1465-0