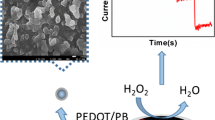
Abstract
We describe an electrochemical sensor for hydrogen peroxide (H2O2) that is making use of Prussian Blue (PB) electrodeposited on a macroporous (mp) gold skeleton electrode. An mp-Cu film was first prepared as a template and the converted into an mp-Au film through a replacement reaction without destructing the structure. Next, a layer of PB was electrochemically deposited on the surface of the mp-Au film. The surface morphology of the electrode was characterized by scanning electron microscopy. Attenuated total reflection infrared spectroscopy and X-ray photoelectron spectroscopy were applied to confirm the structural features. The mp-PB/Au film electrode displays high electro-catalytic activity for the reduction of H2O2 at a working potential of −50 mV (vs. Ag/AgCl) and is very stable. It has a linear response to H2O2 in the 50 μM to 11.3 mM concentration range and a sensitivity of 767 μA∙mM−1 cm−2. The electrode also revealed good selectivity in the presence of electro-active species such as ascorbic acid and uric acid.

An electrochemical sensor for determination of hydrogen peroxide was fabricated by electrochemical deposition of Prussian Blue on the surface of a macroporous gold electrode.






Similar content being viewed by others
References
Gong H, Sun M, Fan R, Qian L (2013) One-step preparation of a composite consisting of graphene oxide, Prussian blue and chitosan for electrochemical sensing of hydrogen peroxide. Microchim Acta 180:295–301. doi:10.1007/s00604-012-0929-8
Lin M, Yang J, Cho M, Lee Y (2011) Hydrogen peroxide detection using a polypyrrole/Prussian Blue nanowire modified electrode. Macromol Res 19:673–678. doi:10.1007/s13233-011-0707-1
Doroftei F, Pinteala T, Arvinte A (2014) Enhanced stability of a Prussian blue/sol–gel composite for electrochemical determination of hydrogen peroxide. Microchim Acta 181:111–120. doi:10.1007/s00604-013-1076-6
Ricci F, Amine A, Tuta CS, Ciucu AA, Lucarelli F, Palleschi G, Moscone D (2003) Prussian Blue and enzyme bulk-modified screen-printed electrodes for hydrogen peroxide and glucose determination with improved storage and operational stability. Anal Chim Acta 485:111–120. doi:10.1016/S0003-2670(03)00403-3
Liu Y, Chu Z, Zhang Y, Jin W (2009) Amperometric glucose biosensor with high sensitivity based on self-assembled Prussian Blue modified electrode. Electrochim Acta 54:7490–7494. doi:10.1016/j.electacta.2009.08.002
Jia FL, Yu CF, Gong JM, Zhang LZ (2008) Deposition of Prussian blue on nanoporous gold film electrode and its electrocatalytic reduction of H2O2. J Solid State Electron 12:1567–1571. doi:10.1007/s10008-008-0521-7
Chai GS, Yoon SB, Yu JS, Choi JH, Sung YE (2004) Ordered porous carbons with tunable pore sizes as catalyst supports in direct methanol fuel cell. J Phys Chem B 108:7074–7079. doi:10.1021/jp0370472
Chen X, Chen Z, Tian R, Yan W, Yao C (2012) Glucose biosensor based on three dimensional ordered macroporous self-doped polyaniline/Prussian blue bicomponent film. Anal Chim Acta 723:94–100. doi:10.1016/j.aca.2012.02.032
Paula RM, Pallone EMJA, Neves S (2006) A new approach to obtain lithium nickel cobalt oxide porous films. Electrochim Acta 51:6419–6425. doi:10.1016/j.electacta.2006.04.026
Shin HC, Dong J, Liu ML (2003) Nanoporous structures prepared by an electrochemical deposition process. Adv Mater 15:1610–1614. doi:10.1002/adma.200305160
Li Y, Jia WZ, Song YY, Xia XH (2007) Superhydrophobicity of 3D porous copper films prepared using the hydrogen bubble dynamic template. Chem Mater 19:5758–5764. doi:10.1021/cm071738j
Li Y, Song YY, Yang C, Xia XH (2007) Hydrogen bubble dynamic template synthesis of porous gold for nonenzymatic electrochemical detection of glucose. Electrochem Commun 9:981–988. doi:10.1016/j.elecom.2006.11.035
Cherevko S, Chung CH (2010) The porous CuO electrode fabricated by hydrogen bubble evolution and its application to highly sensitive non-enzymatic glucose detection. Talanta 80:1371–1377. doi:10.1016/j.talanta.2009.09.038
Ahmadalinezhad A, Kafi AKM, Chen A (2009) Glucose biosensing based on the highly efficient immobilization of glucose oxidase on a Prussian blue modified nanostructured Au surface. Electrochem Commun 11:2048–2051. doi:10.1016/j.elecom.2009.08.048
Chen S, Yuan R, Chai Y, Hu F (2013) Electrochemical sensing of hydrogen peroxide using metal nanoparticles: a review. Microchim Acta 180:15–32. doi:10.1007/s00604-012-0904-4
Sanghavi BJ, Wolfbeis OS, Hirsch T, Swami NS Nanomaterial-based electrochemical sensing of neurological drugs and neurotransmitters. Microchim Acta doi: 10.1007/s00604-014-1308-4
Zhao G, Feng JJ, Zhang QL, Li SP, Chen HY (2005) Synthesis and characterization of Prussian Blue modified magnetite nanoparticles and its application to the electrocatalytic reduction of H2O2. Chem Mater 17:3154–3159. doi:10.1021/cm048078s
Lisowska-Oleksiak A, Nowak AP, Jasulaitiene V (2006) Poly(3,4-ethylenedioxythiophene)-Prussian Blue hybrid material: evidence of direct chemical interaction between PB and pEDOT. Electrochem Commun 8:107–112. doi:10.1016/j.elecom.2005.10.028
Karyakin AA, Karyakina EE, Gorton L (1999) On the mechanism of H2O2 reduction at Prussian Blue modified electrodes. Electrochem Commun 1:78–82. doi:10.1016/S1388-2481(99)00010-7
Yu H, Sheng QL, Li L, Zheng JB (2007) Rapid electrochemical preparation of a compact and thick Prussian blue film on composite ceramic carbon electrode from single ferricyanide solution in the presence of HAuCl4. J Electroanal Chem 606:55–62. doi:10.1016/j.jelechem.2007.04.014
Jin E, Lu X, Cui L, Chao D, Wang C (2010) Fabrication of graphene/prussian blue composite nanosheets and their electrocatalytic reduction of H2O2. Electrochim Acta 55:7230–7234. doi:10.1016/j.electacta.2010.07.029
Du D, Wang MH, Qin YH, Lin YH (2010) One-step electrochemical deposition of Prussian Blue–multiwalled carbon nanotube nanocomposite thin-film: preparation, characterization and evaluation for H2O2 sensing. J Mater Chem 20:1532–1537. doi:10.1039/B919500A
Razmi H, Mohammad-Rezaei R, Heidari H (2009) Self-assembled Prussian Blue nanoparticles based electrochemical sensor for high sensitive determination of H2O2 in acidic media. Electroanalysis 21:2355–2362. doi:10.1002/elan.200904687
Zou YJ, Sun LX, Xu F (2007) Prussian Blue electrodeposited on MWNTs–PANI hybrid composites for H2O2 detection. Talanta 72:437–442. doi:10.1016/j.talanta.2006.11.001
Kumar SS, Joseph J, Phani KL (2007) Novel method for deposition of gold-prussian blue nanocomposite films induced by electrochemically formed gold nanoparticles: characterization and application to electrocatalysis. Chem Mater 19:4722–4730. doi:10.1021/cm050929o
Li NB, Park JH, Park K, Kwon SJ, Shin H, Kwak J (2008) Characterization and electrocatalytic properties of Prussian blue electrochemically deposited on nano-Au/PAMAM dendrimer-modified gold electrode. Biosens Bioelectron 23:1519–1526. doi:10.1016/j.bios.2008.01.009
Acknowledgments
This work was supported by Ministry of Science, ICT & Future Planning of Korea (Grant no. 2012–002285) and Basic Science Research Program through the National Research Foundation of Korea Grant funded by the Ministry of Science, ICT & Future Planning (2009–0083540).
Author information
Authors and Affiliations
Corresponding author
Additional information
Jiao Yang and Meng Lin equally contributed to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOC 189 kb)
Rights and permissions
About this article
Cite this article
Yang, J., Lin, M., Cho, M. et al. Determination of hydrogen peroxide using a Prussian Blue modified macroporous gold electrode. Microchim Acta 182, 1089–1094 (2015). https://doi.org/10.1007/s00604-014-1433-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00604-014-1433-0