Abstract
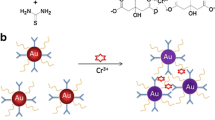
We are presenting a colorimetric assay for mercury (II) ions. It is based on citosan-functionalized gold nanoparticles (AuNPs) that act as a signaling probe. Hg (II) induces the aggregation of the chitosan-AuNPs through a chelation reaction that occurs between chitosan and Hg (II). This results in a strong decrease of the absorbance of the modified AuNPs and a color change from red to blue. This sensing system displays excellent selectivity over other metal ions and a detection limit as low as 1.35 μM which is lower than the allowed level of Hg (II) in drinking water (30 μM) as defined by World Health Organization. The method is inexpensive, facile, sensitive, and does not require the addition of other reagents in order to improving sensitivity.

An inexpensive, facile, and ssensitive, colorimetric method for the detection of Hg (II) is presented.




Similar content being viewed by others
References
Harris HH, Pickering IJ, George GN (2003) The chemical form of mercury in fish. Science 301:1203
Clarkson TW, Magos L, Myers GJ (2003) The toxicology of mercury–current exposures and clinical manifestations. N Engl J Med 349:1731–1737
Morel FMM, Kraepiel AML, Amyot M (1998) The chemical cycle and bioaccumulation of mercury. Annu Rev Ecol Syst 29:543–566
Moreton JA, Delves HTJ (1998) Simple direct method for the determination of total mercury levels in blood and urine and nitric acid digests of fish by inductively coupled plasma mass spectrometry. Anal Atom Spectrom 13:659–665
Wan CC, Chen CS, Jiang SJ (1997) Determination of mercury compounds in water samples by liquid chromatography inductively coupled plasma mass spectrometry with an in situ nebulizer/vapor generator. J Anal At Spectrom 12:683–687
Geng W, Nakajima T, Takanashi H, Ohki A (2008) Determination of mercury in ash and soil samples by oxygen flask combustion method-cold vapor atomic fluorescence spectrometry (CVAFS). J Hazard Mater 154:325–30
Nolan EM, Lippard SJA (2003) “Turn-on” fluorescent sensor for detection of mercuric ion in aqueous media. J Am Chem Soc 125:14270–14271
Kim IB, Bunz UHF (2006) Modulating the sensory response of a conjugated polymer by proteins: an agglutination assay for mercury ions in water. J Am Chem Soc 128:2818–2819
Miyake Y, Togashi H, Tashiro M, Yamaguchi H, Oda S, Kudo M, Tanaka Y, Kondo Y, Sawa R, Fujimoto T, Machinami T, Ono A (2006) J Am Chem Soc 128:2172
Liu J, Lu Y (2007) Rational design of “turn-on” allosteric DNAzyme catalytic beacons for aqueous mercury ions with ultrahigh sensitivity and selectivity. Angew Chem Int Ed 46:7587–7590
Darbha GK, Singh AK, Rai US, Yu E, Yu H, Ray PC (2008) Highly selective detection of Hg2+ ion using NLO properties of gold nanomaterial. J Am Chem Soc 130:8038–8042
Palomares E, Vilar R, Durrant JR (2004) Heterogeneous colorimetric sensor for mercuric salts. Chem Commun 4:362–363
Guo W, Yuan J, Wang E (2009) Oligonucleotide-stabilized Ag nanoclusters as novel fluorescence probes for the highly selective and sensitive detection of the Hg2+ ion. Chem Commun 23:3395–3397
Rosi NL, Mirkin CA (2005) Nanostructures in biodiagnostics. Chem Rev 105:1547–1562
Zhao WA, Brook MA, Li YF (2008) Design of gold nanoparticle-based colorimetric biosensing assays. ChemBioChem 9:2363–2371
Carey JR, Suslick KS, Hulkower KI, Imlay JA, Imlay KRC, Ingison CK, Ponder JB, Sen A, Wittrig AE (2011) Rapid identification of bacteria with a disposable colorimetric sensor array. J Am Chem Soc 133:7571–7576
Laurieri N, Crawford MHJ, Kawamura A, Westwood IM, Robinson J, Fletcher AM, Davies SG, Sim E, Russell AJ (2010) J Am Chem Soc 132:3238
Sardar R, Rark JW, Shumaker-Parry JS (2007) Polymer-induced synthesis of stable gold and silver nanoparticles and subsequent ligand exchange in water. Langmuir 23:11883–11889
Huang CC, Li YF, Cao ZH, Tan WH, Chang HT (2005) Aptamer-modified gold nanoparticles for colorimetric determination of platelet-derived growth factors and their receptors. Anal Chem 77:5735–5741
Haiss W, Thanh NTK, Aveyard J, Fernig DG (2007) Determination of size and concentration of gold nanoparticles from UV–vis spectra. Anal Chem 79:4215–4221
Li L, Li B, Cheng D, Mao L (2010) Visual detection of melamine in raw milk using gold nanoparticles as colorimetric probe. Food Chem 122:895–900
Ma Y, Jiang L, Mei Y, Song R, Tian D, Huang H (2013) Colorimetric sensing strategy for mercury(II) and melamine utilizing cysteamine-modified gold nanoparticles. Analyst 138:5338–5343
Du JJ, Yin SY, Jiang L, Ma B, Chen XD (2013) A colorimetric logic gate based on free gold nanoparticles and the coordination strategy between melamine and mercury ions. Chem Commun 49:4196–4198
Tan DD, He Y, Xing XJ, Zhao Y, Tang HW, Pang DW (2013) Aptamer functionalized gold nanoparticles based fluorescent probe for the detection of mercury (II) ion in aqueous solution. Talanta 113:26–30
Homraruen D, Sirijindalert T, Dubas L, Sukwattanasinitt M, Ajavakom A (2013) Selective fluorescent sensor for mercury ions in aqueous media using a 1,4-dihydropyridine derivative. Tetrahedron 69:1617–1621
Hu B, Hu LL, Chen ML, Wang JH (2013) A FRET ratiometric fluorescence sensing system for mercury detection and intracellular colorimetric imaging in live Hela cells. Biosens Bioelectron 49:499–505
Zhang YQ, Gao LJ, Wen LP, Heng LP, Song YL (2013) Highly sensitive, selective and reusable mercury(II) ion sensor based on a ssDNA-functionalized photonic crystal film. Phys Chem Chem Phys 15:11943–11949
Acknowledgments
All authors gratefully acknowledge the financial support of Scientific Research Project of Beijing Educational Committee (KM201410028006) and the Natural Science Foundation of China (No. 21371123).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chen, Z., Zhang, C., Tan, Y. et al. Chitosan-functionalized gold nanoparticles for colorimetric detection of mercury ions based on chelation-induced aggregation. Microchim Acta 182, 611–616 (2015). https://doi.org/10.1007/s00604-014-1365-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00604-014-1365-8